
Which compound exhibits maximum dipole moment among the following?
(A)
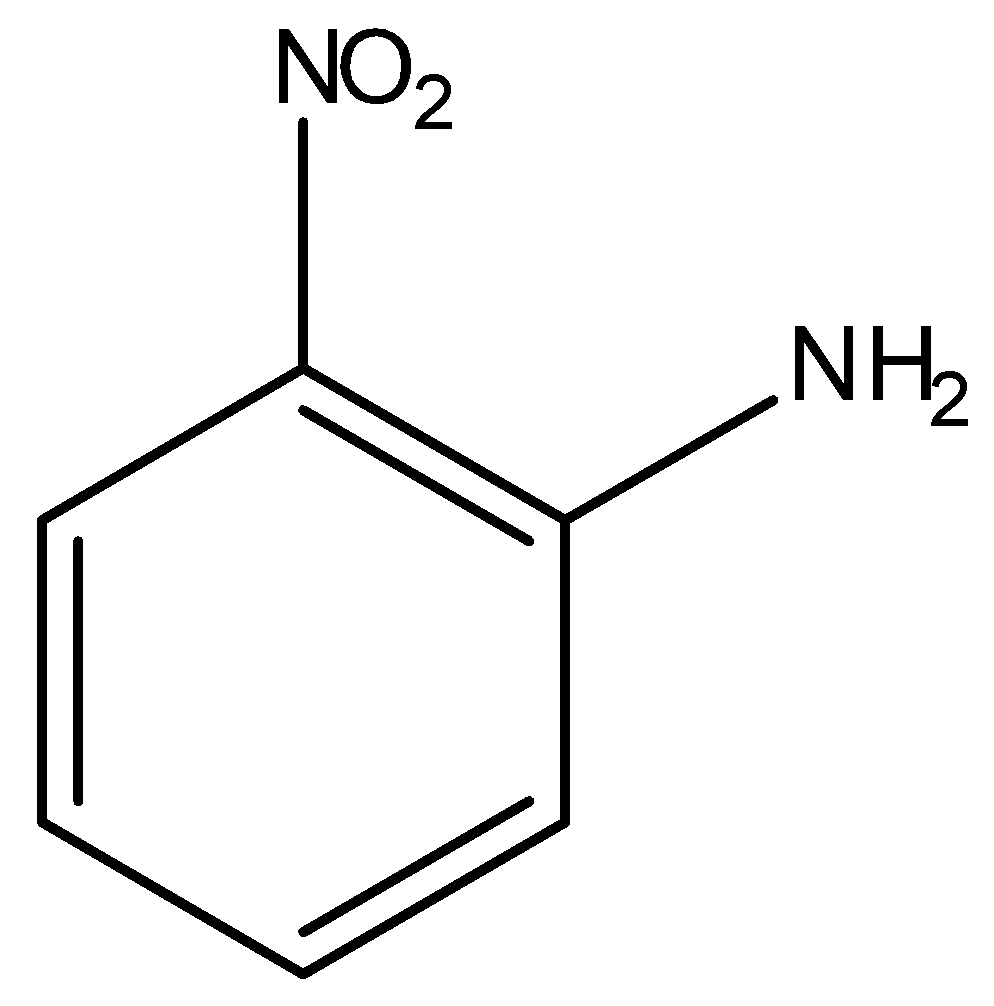
(B)
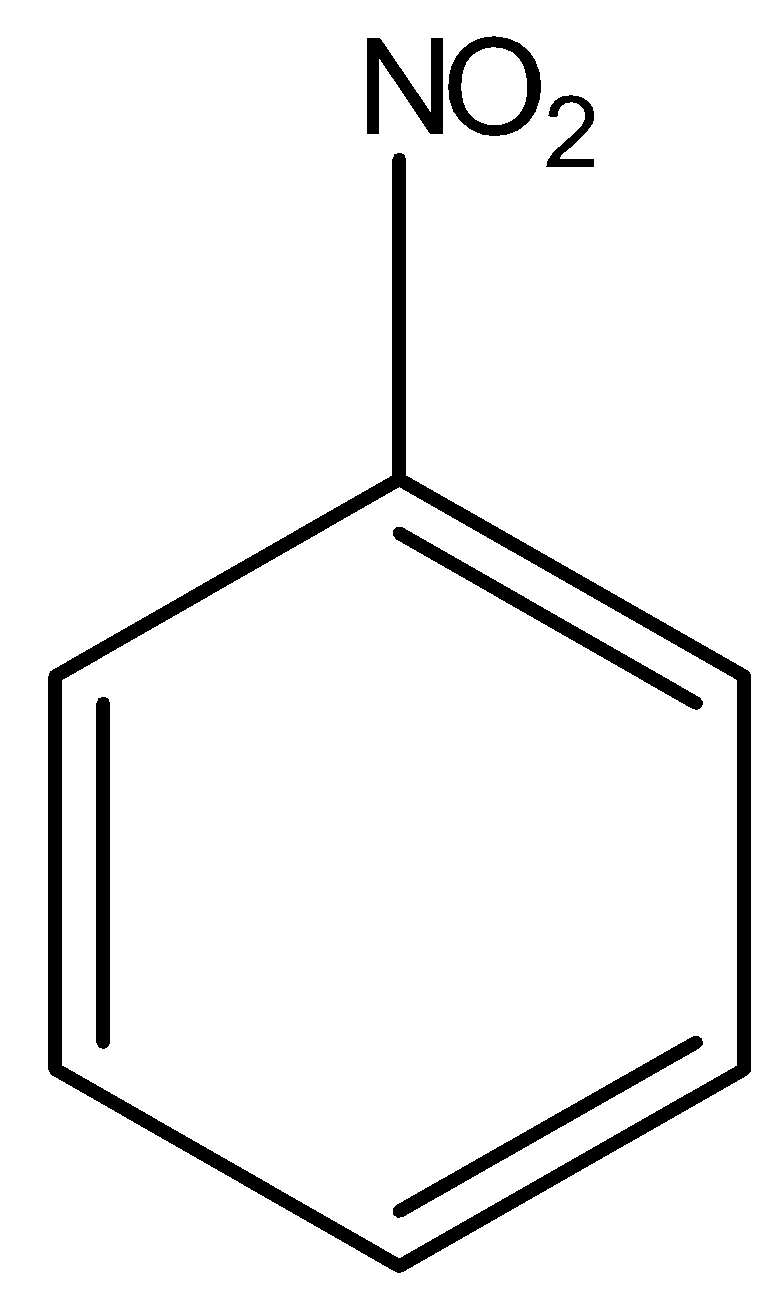
(C)
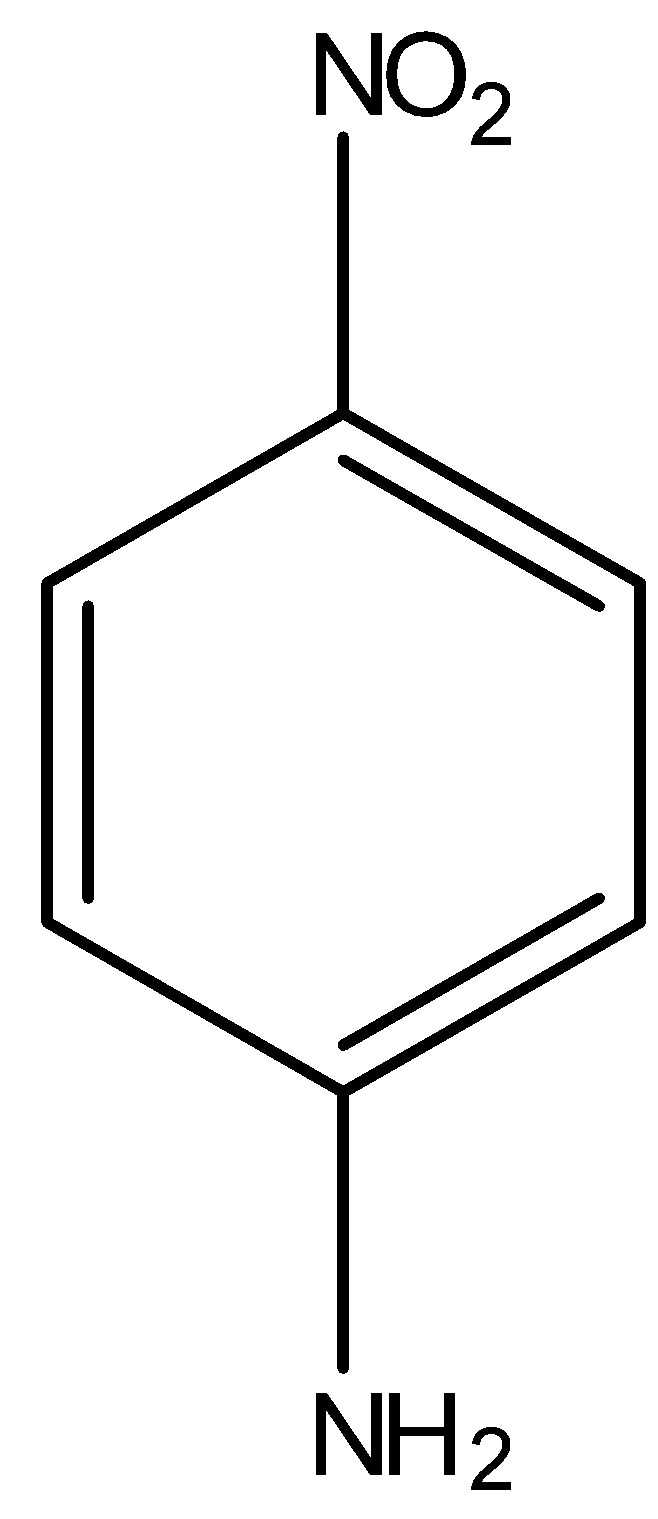
(D)
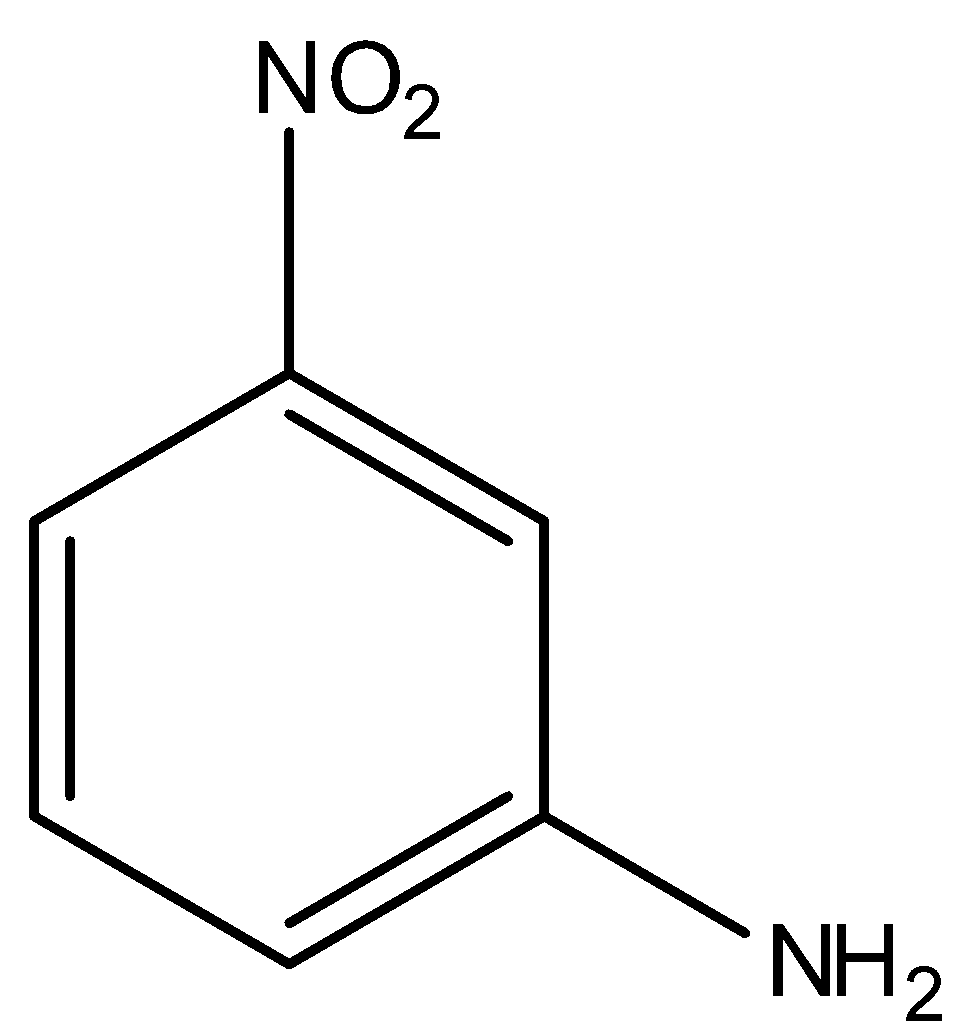




Answer
578.7k+ views
Hint: You must know that $N{H_2}$ group is an electron donating group while $N{O_2}$ group is an electron withdrawing group. So, if an $N{H_2}$ group is present on a ring, then electron density will be towards the ring and when an $N{O_2}$ group is present on a ring, then electron density will be towards the $N{O_2}$ group. Dipole moment is the measure of the system's overall polarity.
Complete step by step solution:
Dipole moment, denoted by the symbol $\mu $, arises in any system when there is a separation of negative and positive charges. Separation of charges occurs because of the electronegativity difference between two chemically bonded atoms. Thus, dipole moment is a measure of the polarity of a system. Dipole moment is represented by an arrow with the arrowhead on the negative centre and cross on the positive centre. Arrow symbolises the shift of electron density in the molecule. Now, in the question, we are given nitrobenzene and aniline derivatives basically. Dipole moment in some of the following compounds:
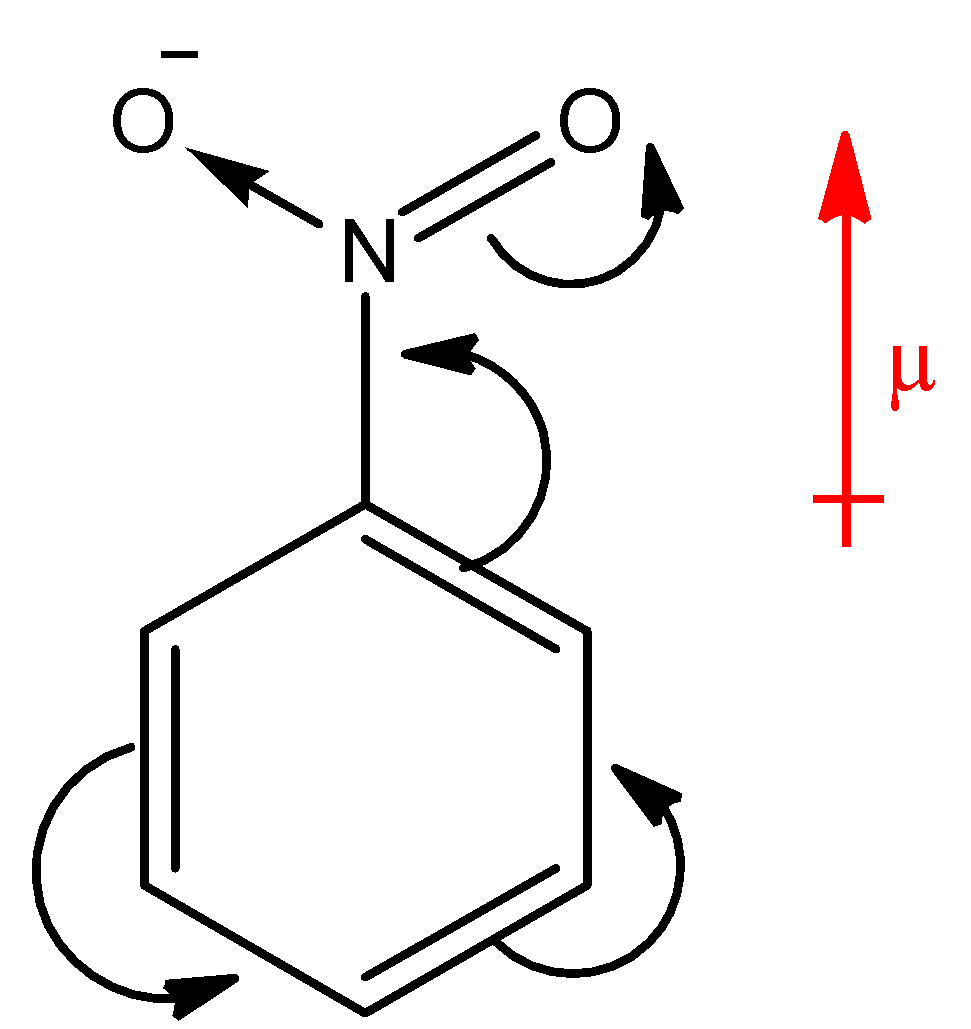
Above compound is nitro benzene. Here, $N{O_2}$ group is present on a ring which is an electron withdrawing group, hence the electron density will move towards the $N{O_2}$ group. There will be separation of charges in the system; the ring will acquire partial positive charge and the nitro group will acquire partial negative charge. Hence, the overall dipole moment ($\mu $) of the compound will be in upward direction as shown in the diagram with red arrow. Whereas, the reverse of nitro benzene happens in aniline as shown in the below diagram.
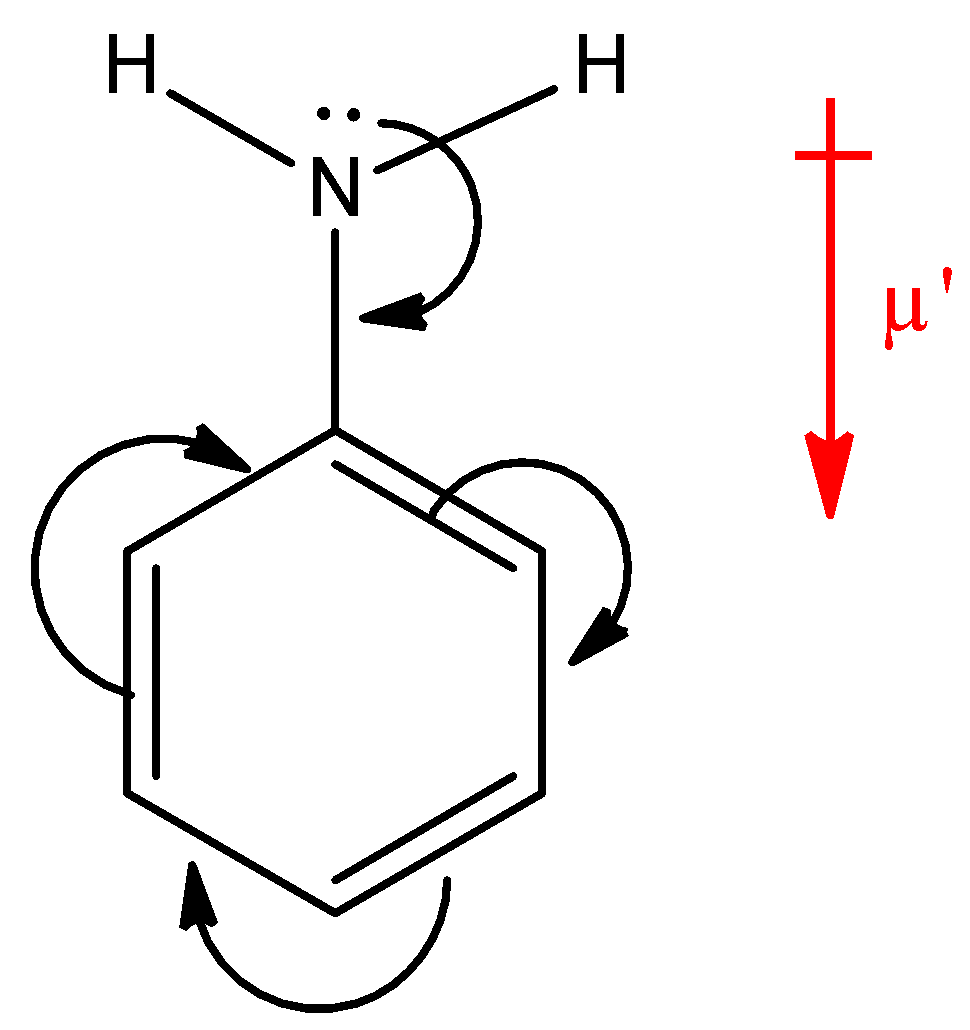
Here, $N{H_2}$ group is present on a ring, which is an electron donating group, thus the electron density will be towards the ring in aniline. Hence, the dipole moment ($\mu '$) will be downwards. Now, in p-nitroaniline, where both $N{H_2}$ and $N{O_2}$ group are present:
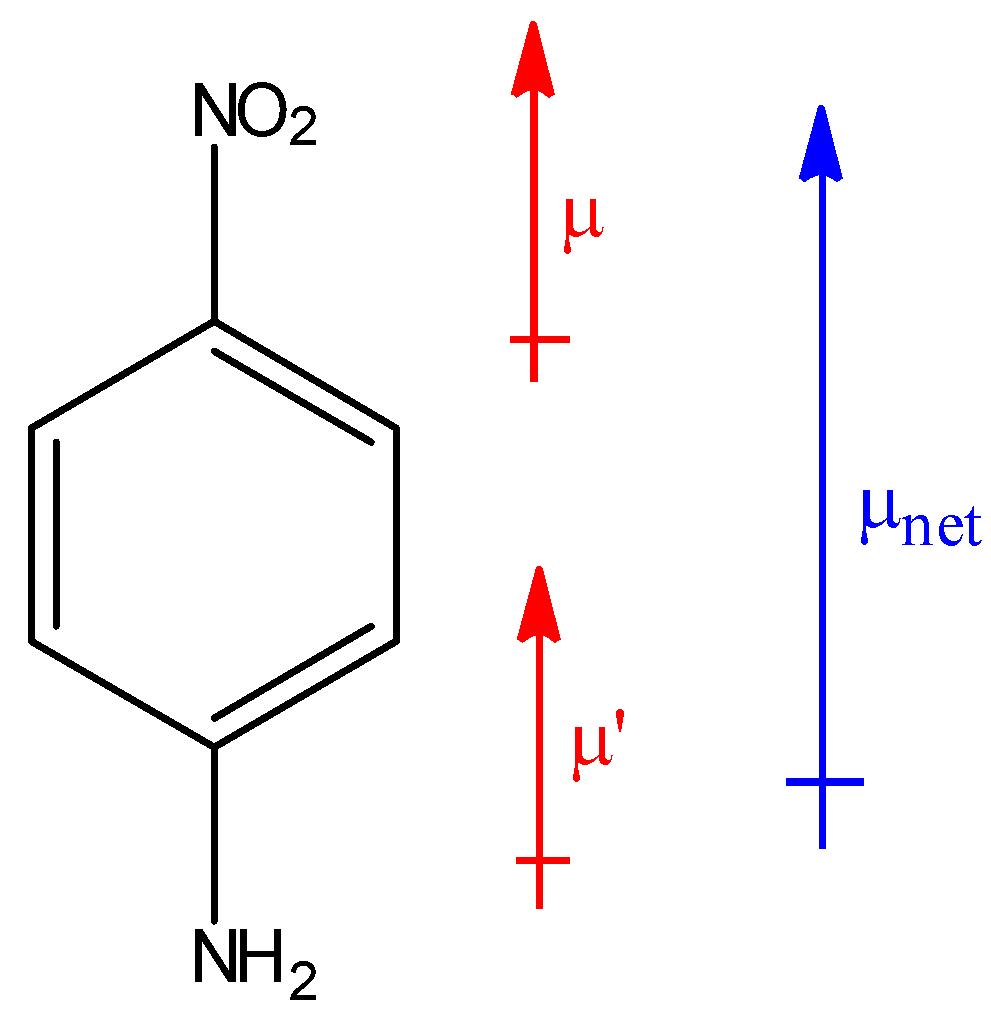
In the above compound, the net resultant dipole moment will be upwards because both individual dipole moments $\mu $ and $\mu '$ are in upward direction. Hence, this compound will have the highest dipole moment among all the given benzene derivatives.
Thus, option C is correct.
Note: Dipole moment is a vector quantity since it has both magnitude and direction. The net resultant dipole moment is the vector sum of all the individual dipole moments in a given system. Value of the dipole moment is zero when two individual dipole moments cancel out each other.
Complete step by step solution:
Dipole moment, denoted by the symbol $\mu $, arises in any system when there is a separation of negative and positive charges. Separation of charges occurs because of the electronegativity difference between two chemically bonded atoms. Thus, dipole moment is a measure of the polarity of a system. Dipole moment is represented by an arrow with the arrowhead on the negative centre and cross on the positive centre. Arrow symbolises the shift of electron density in the molecule. Now, in the question, we are given nitrobenzene and aniline derivatives basically. Dipole moment in some of the following compounds:

Above compound is nitro benzene. Here, $N{O_2}$ group is present on a ring which is an electron withdrawing group, hence the electron density will move towards the $N{O_2}$ group. There will be separation of charges in the system; the ring will acquire partial positive charge and the nitro group will acquire partial negative charge. Hence, the overall dipole moment ($\mu $) of the compound will be in upward direction as shown in the diagram with red arrow. Whereas, the reverse of nitro benzene happens in aniline as shown in the below diagram.

Here, $N{H_2}$ group is present on a ring, which is an electron donating group, thus the electron density will be towards the ring in aniline. Hence, the dipole moment ($\mu '$) will be downwards. Now, in p-nitroaniline, where both $N{H_2}$ and $N{O_2}$ group are present:

In the above compound, the net resultant dipole moment will be upwards because both individual dipole moments $\mu $ and $\mu '$ are in upward direction. Hence, this compound will have the highest dipole moment among all the given benzene derivatives.
Thus, option C is correct.
Note: Dipole moment is a vector quantity since it has both magnitude and direction. The net resultant dipole moment is the vector sum of all the individual dipole moments in a given system. Value of the dipole moment is zero when two individual dipole moments cancel out each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




