
Which compound gives positive iodoform test?
A.2-pentanone
B.Pivaldehyde
C.Benzaldehyde
D.Isobutyl alcohol
Answer
522.4k+ views
Hint: Draw the molecular structure of each of the given options and remember positive iodoform test is given by compounds containing methyl ketones and 2-alkanols.

Complete step by step answer:
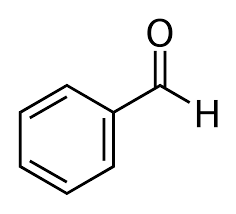
Option (c) Benzaldehyde does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.
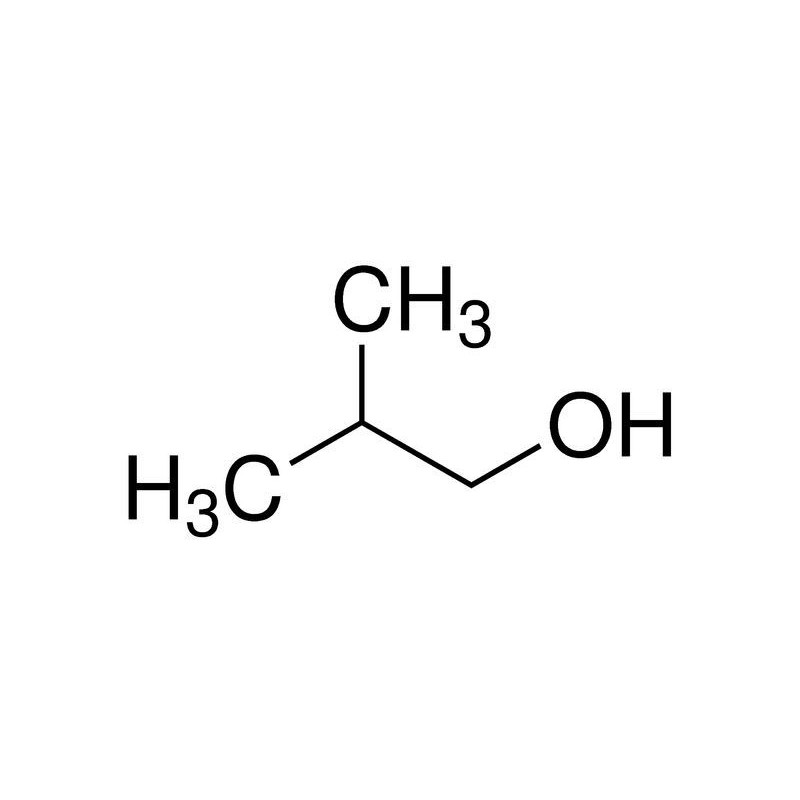
Option (d) Isobutyl alcohol does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.
So, correct option is A
Additional information: The pale yellow precipitate of iodoform ${ (CHI }_{ 3 }{ ) }$ is formed, which can be identified by its characteristic “antiseptic” smell.
Note: If an aldehyde gives a positive iodoform test, then it must be acetaldehyde since it is the only aldehyde with a ${ CH }_{ 3 }CO$ group.

Complete step by step answer:
Iodoform test is used for the identification of aldehyde and ketone.
A positive iodoform test is given by the compounds having ${ CH }_{ 3 }CO$ group in their structure.
When Iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or a secondary alcohol with a methyl group in the alpha position, a pale yellow precipitate of iodoform is formed.
Now we will look at the structure of each of the options provided.
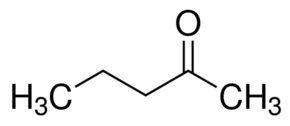
Option (a) 2-pentanone contains a methyl ketone group. Hence, it will give a positive iodoform test.

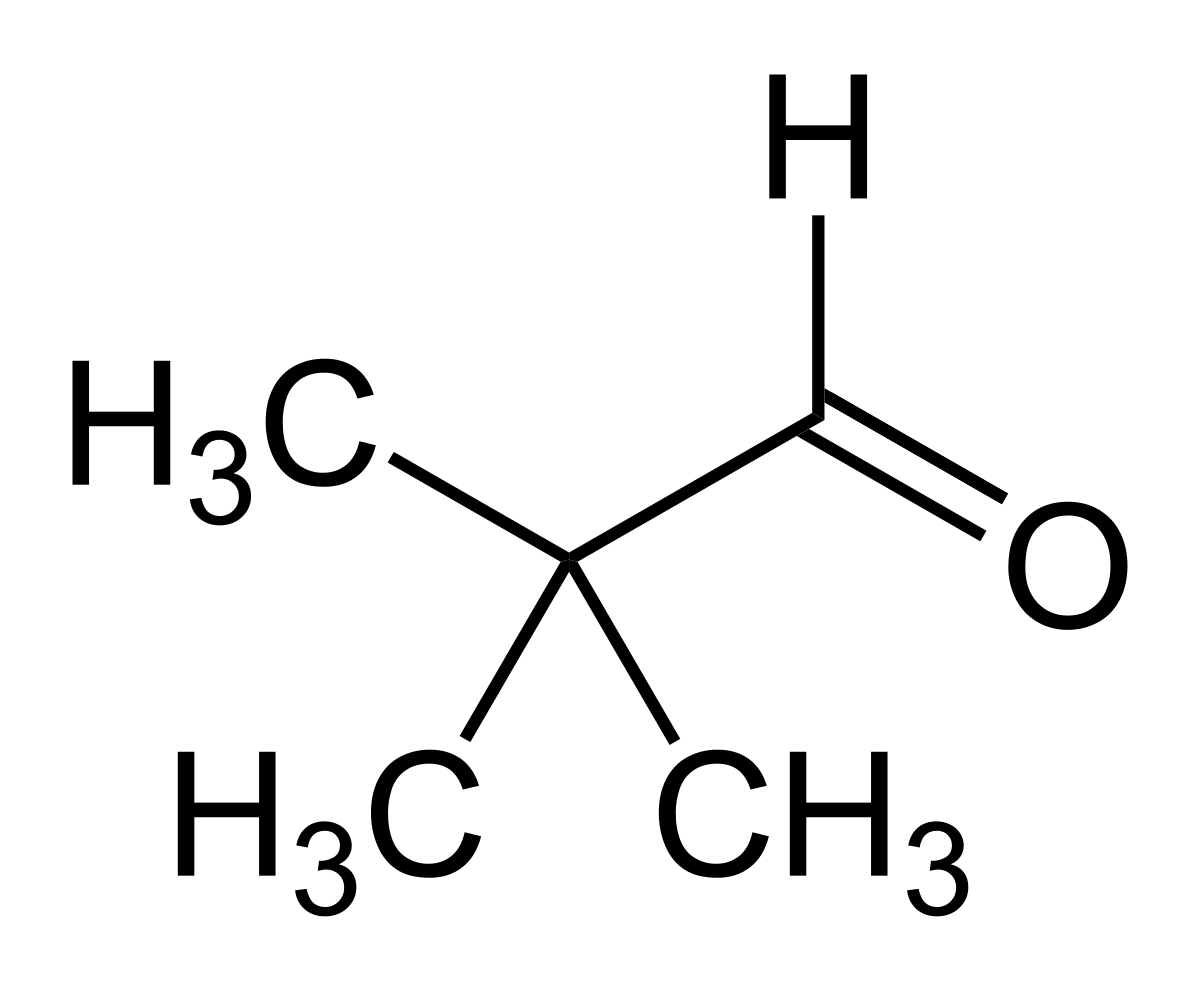
Option (b) Pivaldehyde does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.

Option (c) Benzaldehyde does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.

Option (d) Isobutyl alcohol does not contain methyl ketones and 2-alkanols. That's why it will give a negative test.

So, correct option is A
Additional information: The pale yellow precipitate of iodoform ${ (CHI }_{ 3 }{ ) }$ is formed, which can be identified by its characteristic “antiseptic” smell.
Note: If an aldehyde gives a positive iodoform test, then it must be acetaldehyde since it is the only aldehyde with a ${ CH }_{ 3 }CO$ group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




