
Which compound of xenon is not possible?
A. $Xe{F_2}$
B. $Xe{F_4}$
C. $Xe{F_5}$
D. $Xe{F_6}$
Answer
587.4k+ views
Hint: Xenon is a chemical element with atomic number 54 .It can only combine with an even number of F atoms to form xenon fluorides and not with odd numbers of F atoms.
Complete step by step answer:
Xenon is an inert gas. Its electronic configuration is $[Kr]4{d^{10}}5{s^2}5{p^6}$ . All orbitals that are filled have paired electrons.
Xenon can combine with even number of F atoms to form $Xe{F_2}$ $Xe{F_4}$ and $Xe{F_6}$ .This is because the promotion of 1, 2, or 3 electrons from the 5p filled orbitals to 5d vacant orbitals will give rise to $2,4,6$ half-filled orbitals. The structures are as shown:
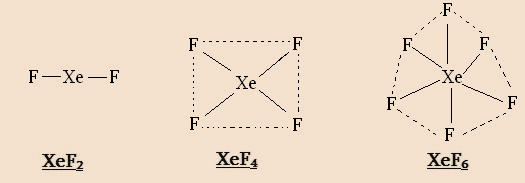
It cannot combine with an odd number of F –atoms.
Thus, the formation of $Xe{F_3}$ and $Xe{F_5}$ is not possible.
Hence, option C is correct.
Note:
Xenon is obtained commercially as a by-product of the separation of air into oxygen and nitrogen. It is 4.5 times heavier than Earth’s atmosphere (which consists of a mixture of a number of gaseous elements and compounds). Its mass comes from its nucleus, which contains 54 protons and a varying (but similar) number of neutrons.
Complete step by step answer:
Xenon is an inert gas. Its electronic configuration is $[Kr]4{d^{10}}5{s^2}5{p^6}$ . All orbitals that are filled have paired electrons.
Xenon can combine with even number of F atoms to form $Xe{F_2}$ $Xe{F_4}$ and $Xe{F_6}$ .This is because the promotion of 1, 2, or 3 electrons from the 5p filled orbitals to 5d vacant orbitals will give rise to $2,4,6$ half-filled orbitals. The structures are as shown:

It cannot combine with an odd number of F –atoms.
Thus, the formation of $Xe{F_3}$ and $Xe{F_5}$ is not possible.
Hence, option C is correct.
Note:
Xenon is obtained commercially as a by-product of the separation of air into oxygen and nitrogen. It is 4.5 times heavier than Earth’s atmosphere (which consists of a mixture of a number of gaseous elements and compounds). Its mass comes from its nucleus, which contains 54 protons and a varying (but similar) number of neutrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




