
Which general formula below represents that of an organic ester?
(A) R-OH
(B) R-COOH
(C) R-O-R
(D) R-COO-R
(E) R-CO-R
Answer
588.9k+ views
Hint: Ester is a chemical compound formed by the reaction between an alcohol and a carboxylic acid. It has a fruity smell and is used in making perfumes, soaps, food flavours, etc.
Complete step by step answer:
-An ester is a chemical compound which has been formed from any organic or inorganic acid where atleast one of the hydroxyl groups (-OH) would be replaced by an (-O-alkyl) group. In simpler words we can say that esters are formed by the substitution reaction of an alcohol and a carboxylic acid.
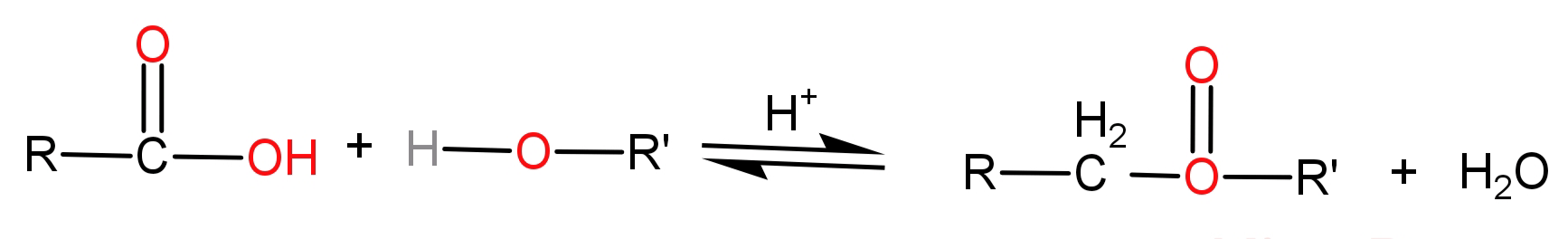
It can also be formed by the reactions of acid halides or acid anhydride with the alcohols and also by reacting salts of carboxylic acids with alkyl halides.
-The general formula of an ester is: R-COO-R’
For example: $C{H_3} - C{H_2} - CO - O - C{H_2} - C{H_3}$ is ethyl propanoate, $H - CO - C - C{H_2} - C{H_2} - C{H_3}$ is propyl methanoate, etc. Let us see their structures also.

-In an ester carbon is bonded to one of the oxygen atoms via a double bond and to the other oxygen atom through a single bond which is further attached to either an alkyl group or an aryl group. In their structure the C-C-O and O-C-O angles measure ${120^ \circ }$.
-The polarity of esters is more than that of ethers but less than that of alcohols. They behave as hydrogen bond acceptors and thus form hydrogen bonds. They are soluble in water and volatile as compared to carboxylic acids.
-Ester has a wide range of applications like:
(1) Since they have fragrant odours they are used in perfumes, essential oils, cosmetics and food flavours also.
(2) It plays the role of an organic solvent.
(3) It is also used in the manufacture of soaps and detergents.
(4) The fatty acid esters of glycerol form natural oils and fats.
(5) They are also found in pheromones.
-Just let us take a look at other options as well options:
(A) R-OH: It is an alcohol.
(B) R-COOH: It is a carboxylic acid.
(C) R-O-R: It is an ester.
(E) R-CO-R: It is a ketone.
So, the correct answer is “Option D”.
Note: A nitrate ester (like nitroglycerine) having a molecular formula of $RON{O_2}$ is highly explosive in nature. It is an ester of nitric acid and alcohol.
Complete step by step answer:
-An ester is a chemical compound which has been formed from any organic or inorganic acid where atleast one of the hydroxyl groups (-OH) would be replaced by an (-O-alkyl) group. In simpler words we can say that esters are formed by the substitution reaction of an alcohol and a carboxylic acid.

It can also be formed by the reactions of acid halides or acid anhydride with the alcohols and also by reacting salts of carboxylic acids with alkyl halides.
-The general formula of an ester is: R-COO-R’
For example: $C{H_3} - C{H_2} - CO - O - C{H_2} - C{H_3}$ is ethyl propanoate, $H - CO - C - C{H_2} - C{H_2} - C{H_3}$ is propyl methanoate, etc. Let us see their structures also.

-In an ester carbon is bonded to one of the oxygen atoms via a double bond and to the other oxygen atom through a single bond which is further attached to either an alkyl group or an aryl group. In their structure the C-C-O and O-C-O angles measure ${120^ \circ }$.
-The polarity of esters is more than that of ethers but less than that of alcohols. They behave as hydrogen bond acceptors and thus form hydrogen bonds. They are soluble in water and volatile as compared to carboxylic acids.
-Ester has a wide range of applications like:
(1) Since they have fragrant odours they are used in perfumes, essential oils, cosmetics and food flavours also.
(2) It plays the role of an organic solvent.
(3) It is also used in the manufacture of soaps and detergents.
(4) The fatty acid esters of glycerol form natural oils and fats.
(5) They are also found in pheromones.
-Just let us take a look at other options as well options:
(A) R-OH: It is an alcohol.
(B) R-COOH: It is a carboxylic acid.
(C) R-O-R: It is an ester.
(E) R-CO-R: It is a ketone.
So, the correct answer is “Option D”.
Note: A nitrate ester (like nitroglycerine) having a molecular formula of $RON{O_2}$ is highly explosive in nature. It is an ester of nitric acid and alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




