
Which graph shows zero activation energy for reaction?
(A)
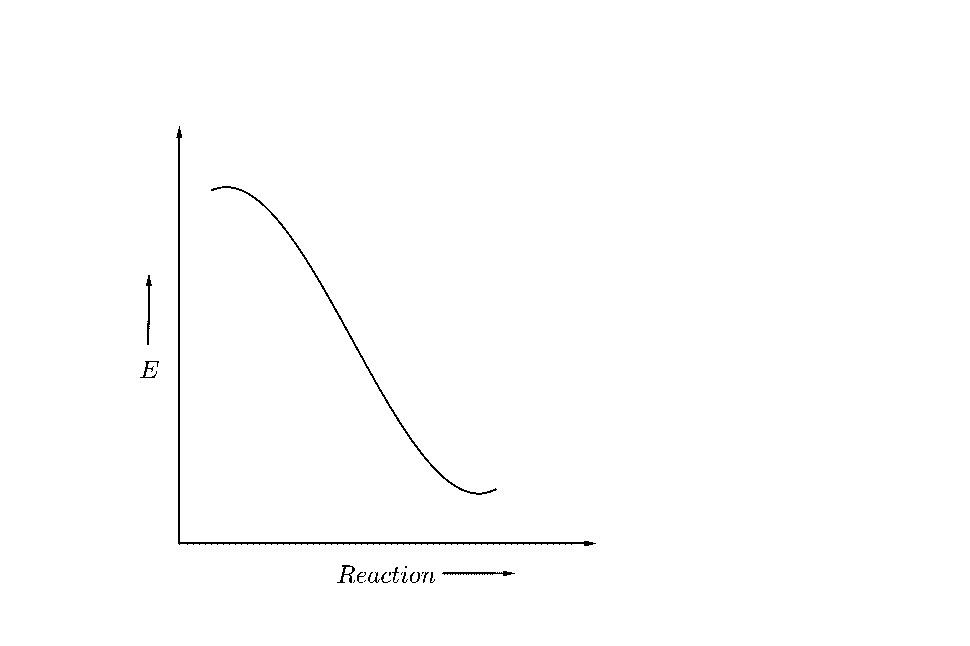
(B)
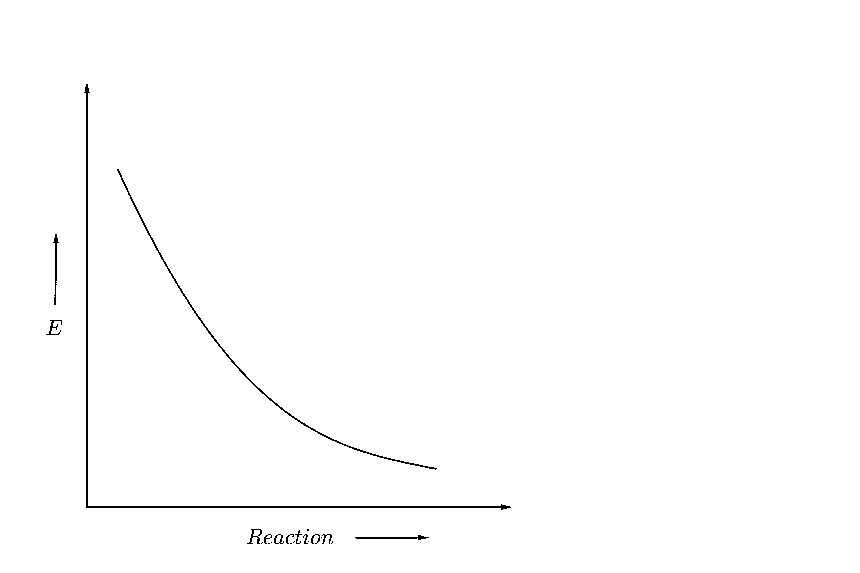
(C)
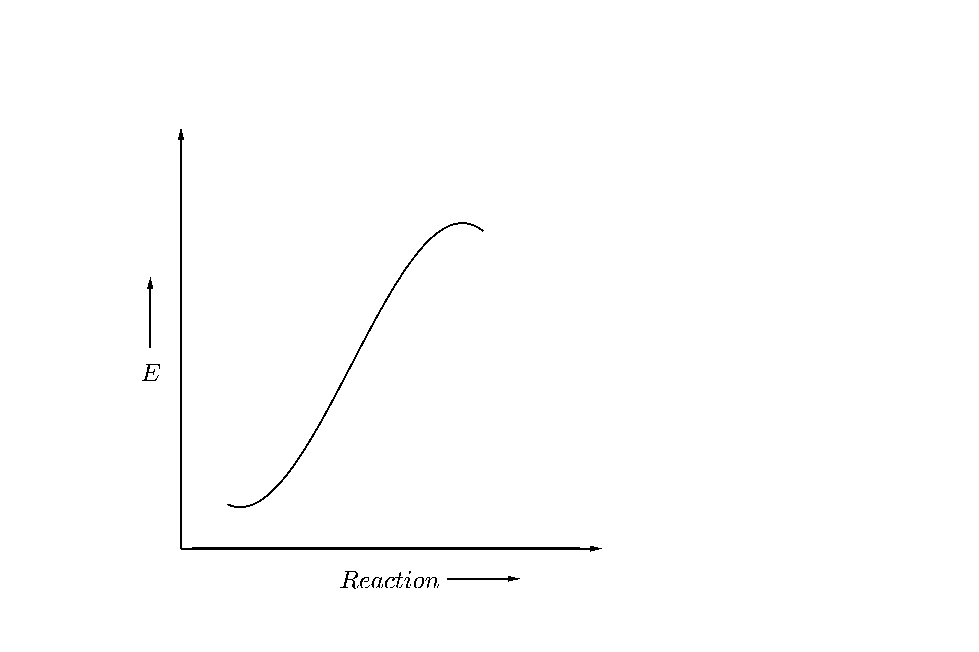
(D)
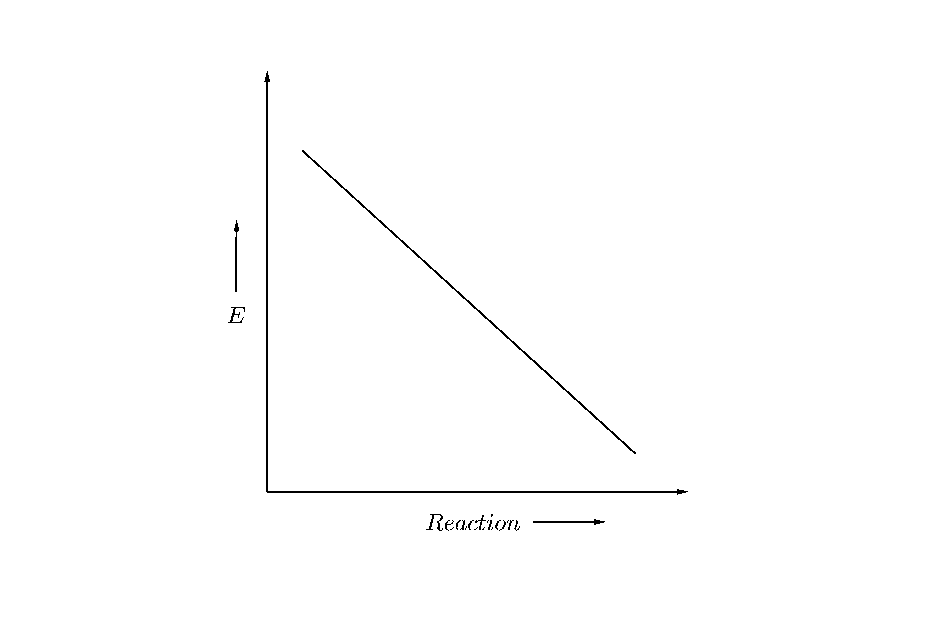




Answer
516.3k+ views
Hint :Activation energy is the energy barrier that a reactant must overcome for forming a product. Zero activation energy means the reactant doesn’t require any more energy to cross the energy barrier, and it can directly get converted to product. This means that the initial energy of the reactant will be equal to the activation energy of the reaction.
Complete Step By Step Answer:
Activation Energy can be defined as the minimum energy required to be provided to the reactants for the successful completion of reaction and formation of products.
It can be understood as an energy barrier, which the reactant molecules need to cross, to convert into products.
The sample graph showing the reactant energy, product energy and the activation energy can be shown as below;
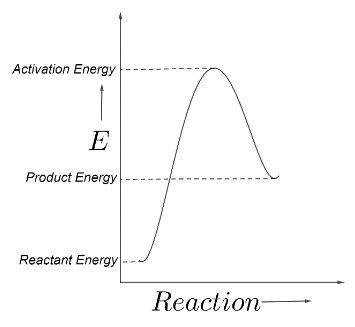
Hence, the energy of the reactants must increase to the activation energy, and after crossing the barrier its energy decreases, because a molecule always tries to reduce its energy for stability.
The activation energy is the difference between the activation energy and the reactant energy.
Now suppose, that the reactant energy is initially equal to the activation energy as shown below
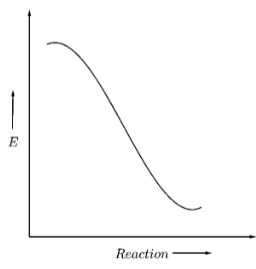
Hence, to form a product, the reactants don’t need to cross any energy barrier, and they can directly convert to product. Hence, the reaction will be spontaneous.
Hence, as the difference between the intermediate energy barrier and the reactant energy is zero, we can say that this graph shows a reaction with zero activation energy.
Note :
Here, we have obtained a graph that shows zero activation energy for a reaction. However this is practically not possible. As per Arrhenius theory, a successful collision of two molecules results in the formation of a product. If the activation energy will be zero, all collisions lead to successful collisions which is not possible. Hence, a reaction with zero activation energy is not possible.
Complete Step By Step Answer:
Activation Energy can be defined as the minimum energy required to be provided to the reactants for the successful completion of reaction and formation of products.
It can be understood as an energy barrier, which the reactant molecules need to cross, to convert into products.
The sample graph showing the reactant energy, product energy and the activation energy can be shown as below;

Hence, the energy of the reactants must increase to the activation energy, and after crossing the barrier its energy decreases, because a molecule always tries to reduce its energy for stability.
The activation energy is the difference between the activation energy and the reactant energy.
Now suppose, that the reactant energy is initially equal to the activation energy as shown below

Hence, to form a product, the reactants don’t need to cross any energy barrier, and they can directly convert to product. Hence, the reaction will be spontaneous.
Hence, as the difference between the intermediate energy barrier and the reactant energy is zero, we can say that this graph shows a reaction with zero activation energy.
Note :
Here, we have obtained a graph that shows zero activation energy for a reaction. However this is practically not possible. As per Arrhenius theory, a successful collision of two molecules results in the formation of a product. If the activation energy will be zero, all collisions lead to successful collisions which is not possible. Hence, a reaction with zero activation energy is not possible.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




