
Which is not the adsorption isobar for chemisorption?
(A)
(B)
(C)
(D)




Answer
584.4k+ views
Hint: Chemisorption is a surface phenomenon. It includes the transfer or sharing of electrons between adsorbates and adsorbents (atoms or molecules). The adsorption of adsorbates on the adsorbents is due to the development of chemical bonds between adsorbate and adsorbent.
Isobaric process is a thermodynamic process where the pressure stays constant.
Complete step by step answer:
From the question we have to find out which graph among the given options is not the adsorption isobar for chemisorption.
We know that in an isobaric process pressure is constant in the process. So, there is no involvement of pressure in the graph.
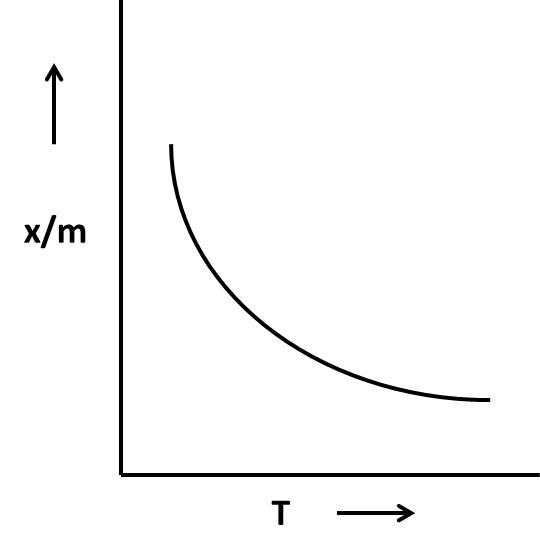
We know that in a physical adsorption the graph shows a decrease in \[\dfrac{x}{m}\] as the temperature (T) rises.
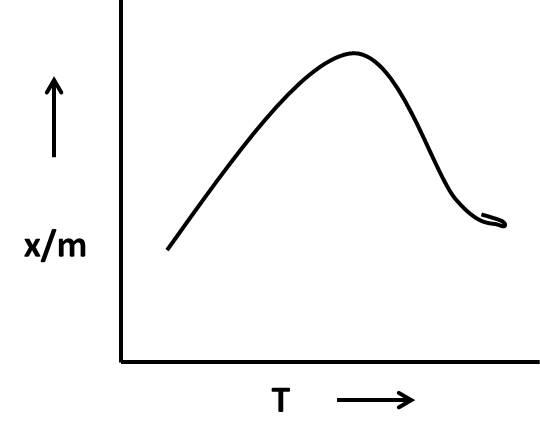
In chemical adsorption reaction, the graph shows an increase in \[\dfrac{x}{m}\]initially and then decreases gradually as the temperature rises.
\[\dfrac{x}{m}\]= rate of adsorption.
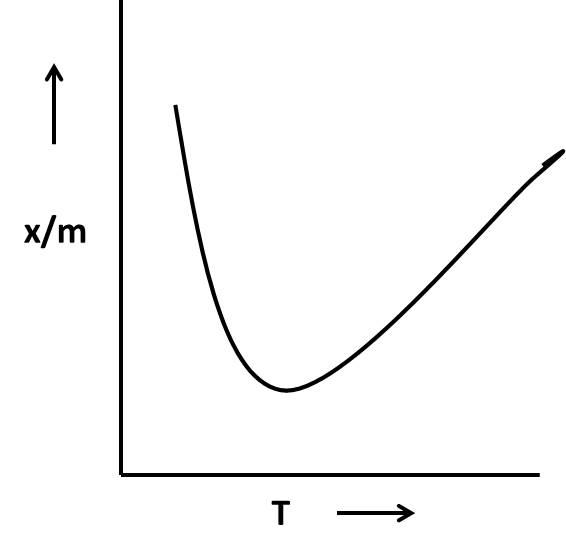
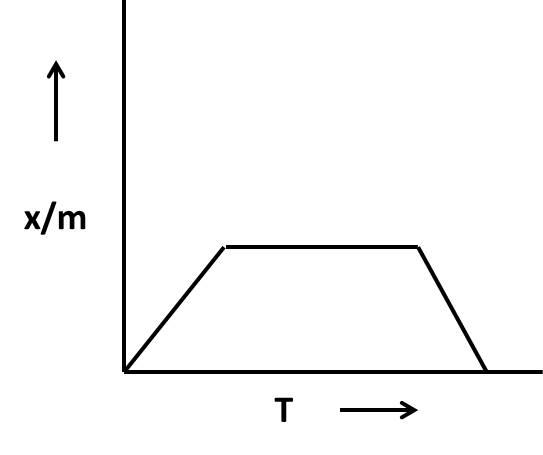
Coming to given options, in the options A and B, there is a decrease in \[\dfrac{x}{m}\]as the temperature rises and in option D, initially \[\dfrac{x}{m}\] increased (designates that the chemical adsorption but the graph shows stability for a long time as the temperature increase (then it is not a chemical adsorption).
As we discussed above in the chemical adsorption graph shows an increase in \[\dfrac{x}{m}\]initially and then decreases gradually as the temperature increases. This trend we can see in option C.
Therefore option C is a suitable graph for the chemisorption process.
So, the graphs not suitable for the adsorption isobar for chemisorption are A, B, and D.
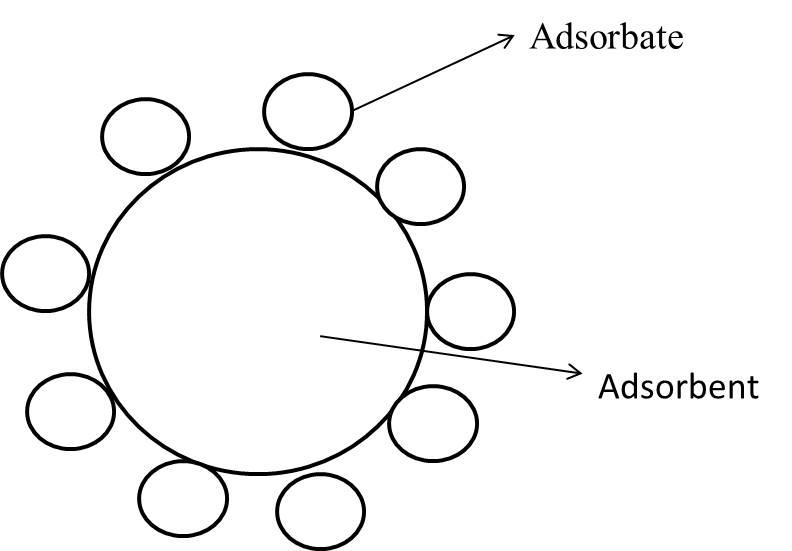
Note: Adsorbent and adsorbate both are not the same.
Adsorbent: An adsorbent is a material which allows a liquid, gas or solid to adhere to its surface.
Adsorbate: A substance that has been adsorbed on the surface of other substances.
Isobaric process is a thermodynamic process where the pressure stays constant.
Complete step by step answer:
From the question we have to find out which graph among the given options is not the adsorption isobar for chemisorption.
We know that in an isobaric process pressure is constant in the process. So, there is no involvement of pressure in the graph.
We know that in a physical adsorption the graph shows a decrease in \[\dfrac{x}{m}\] as the temperature (T) rises.
In chemical adsorption reaction, the graph shows an increase in \[\dfrac{x}{m}\]initially and then decreases gradually as the temperature rises.
\[\dfrac{x}{m}\]= rate of adsorption.
Coming to given options, in the options A and B, there is a decrease in \[\dfrac{x}{m}\]as the temperature rises and in option D, initially \[\dfrac{x}{m}\] increased (designates that the chemical adsorption but the graph shows stability for a long time as the temperature increase (then it is not a chemical adsorption).
As we discussed above in the chemical adsorption graph shows an increase in \[\dfrac{x}{m}\]initially and then decreases gradually as the temperature increases. This trend we can see in option C.
Therefore option C is a suitable graph for the chemisorption process.
So, the graphs not suitable for the adsorption isobar for chemisorption are A, B, and D.
Note: Adsorbent and adsorbate both are not the same.
Adsorbent: An adsorbent is a material which allows a liquid, gas or solid to adhere to its surface.
Adsorbate: A substance that has been adsorbed on the surface of other substances.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




