
Which is the most stable conformer of ethane-1,2-diol?
A)

B)

C)
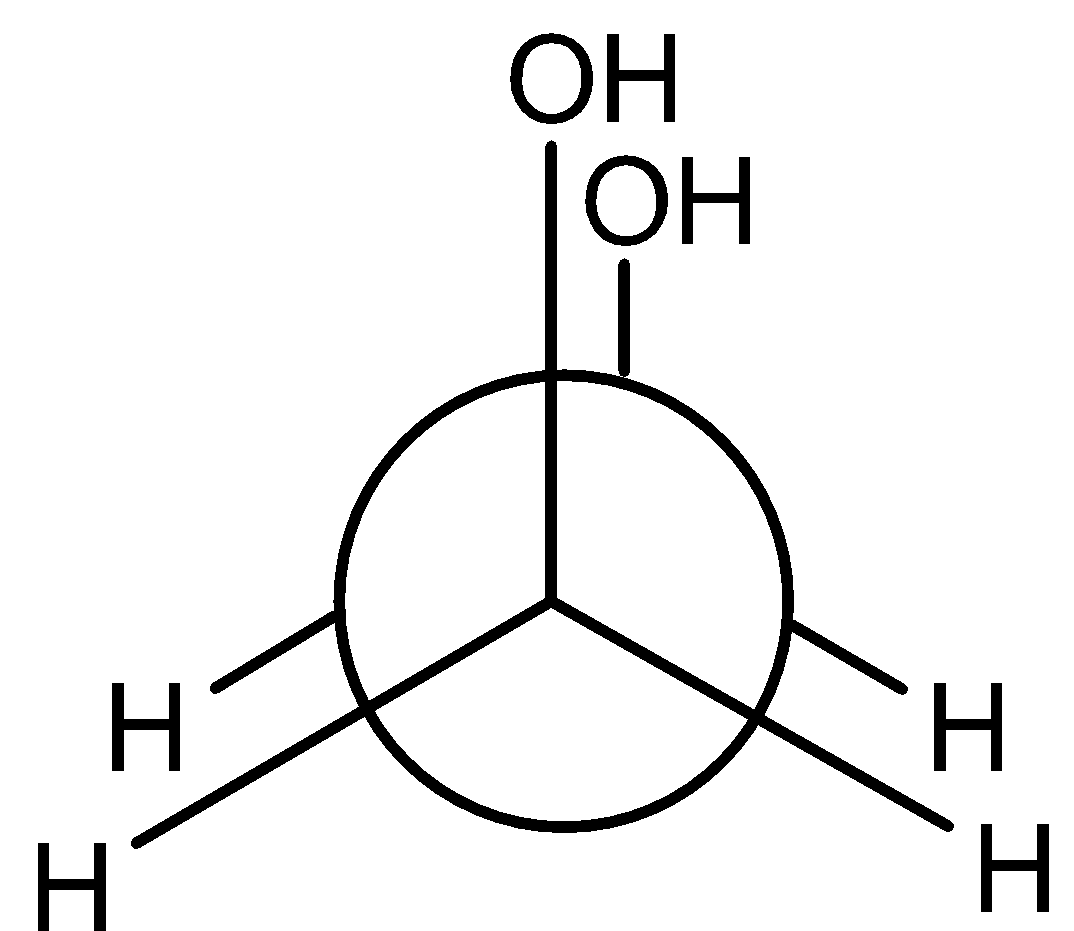
D)
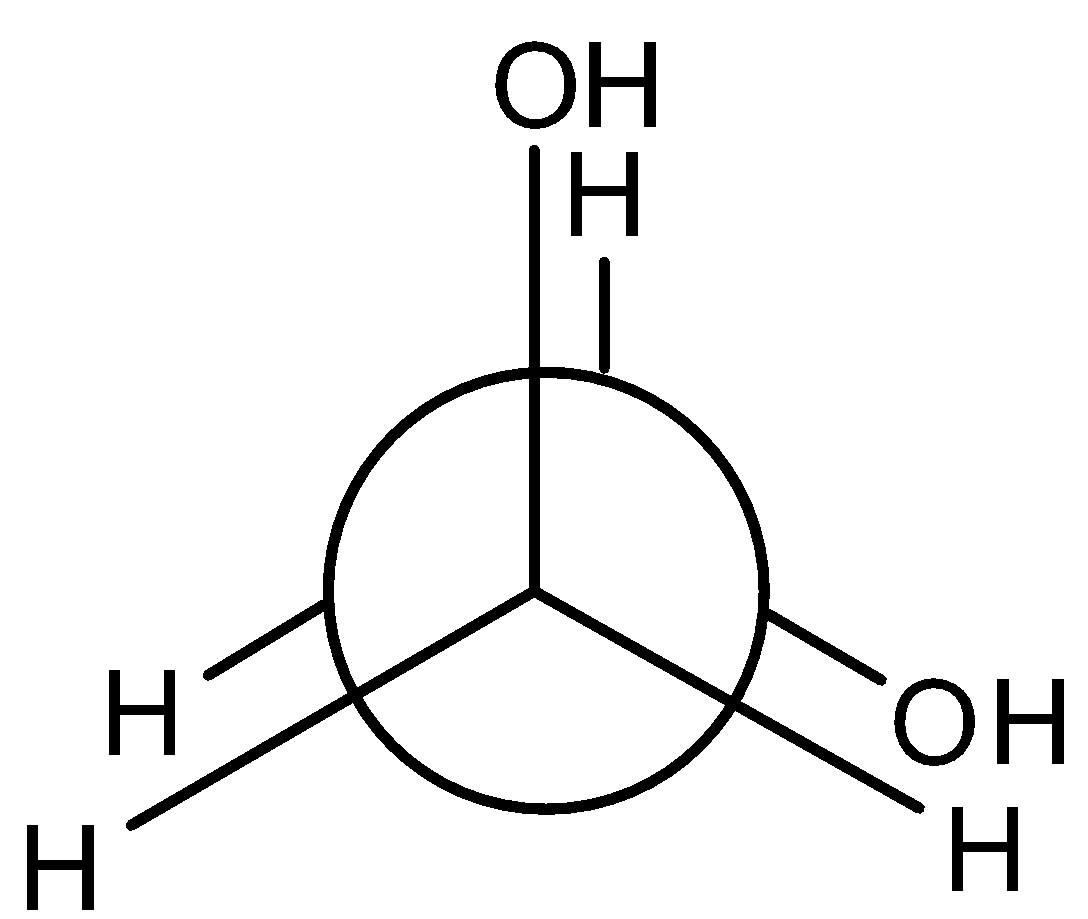




Answer
543.3k+ views
Hint: The answer here is based on the stereochemistry of the molecules given where the stability of the conformer is dependent on the hydrogen bonding and the torsional strain factor also plays role in this.
Complete step by step answer:
In the lower classes, we have studied the concept called stereochemistry in the organic chemistry section which tells in detail about the arrangement of atoms in space.
We shall see in detail the stability of the conformers to deduce the required answer.
- Stereochemistry is the study of the molecules in space which tells about the arrangement of atoms in a molecule in space. Thus, this is also called 3D chemistry as the molecules are studied in the 3-dimensional form.
- Stability of the conformer is based on the Gauche form or the anti-form of the conformer.
- generally, the Gauche form is less stable compared to the anti staggered form. But, when the given compound is showing an intramolecular hydrogen bonding or ion-dipole interaction in Gauche form then this form will be more stable than the anti staggered form.
- Here, in ethane-1,2-diol, the Gauche form is more stable due to the intramolecular hydrogen bonding present in it.
- This is shown in the diagram below:
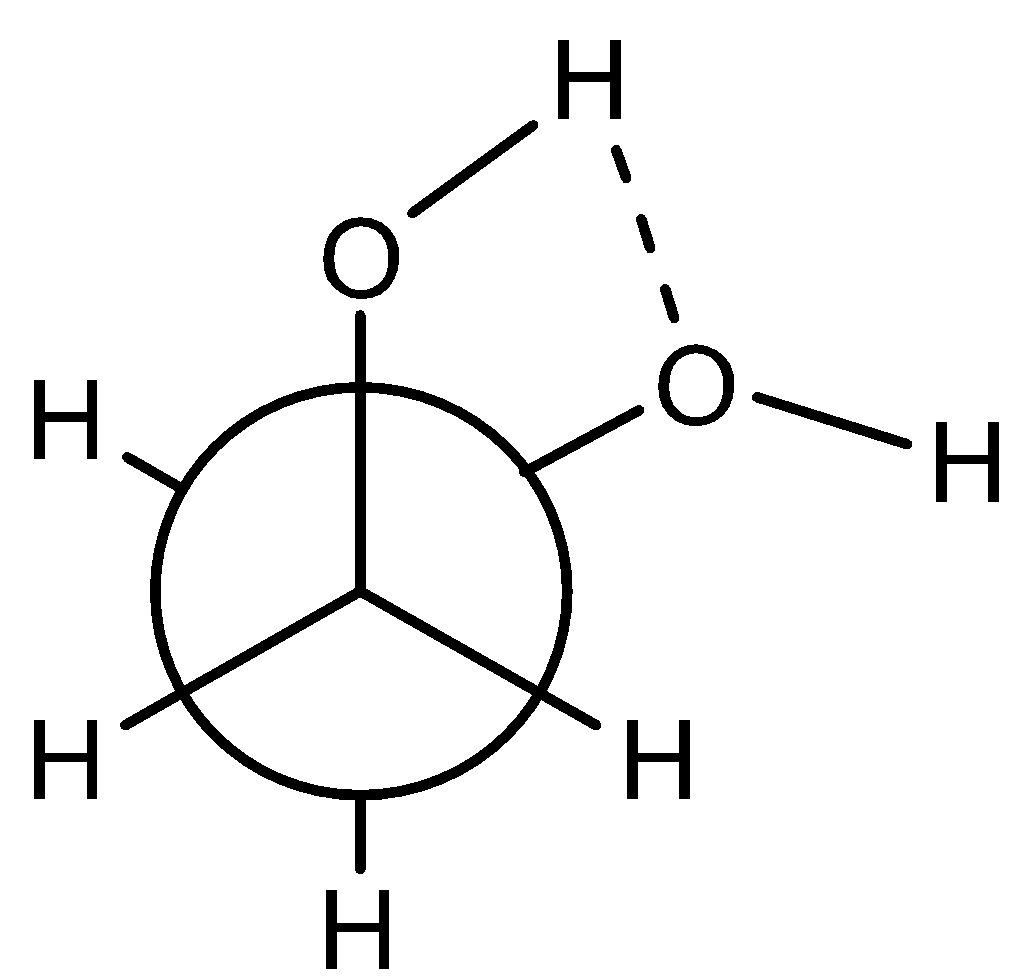
Hence, this intramolecular hydrogen bonding causes this conformation to be more stable.
So, the correct answer is “Option B”.
Note: The Gauche form of the conformer is the one in which the larger atoms are staggered adjacent to each other whereas the anti staggered or anti conformer is the one in which the larger atoms are opposite to each other that is at the angle of ${{180}^{0}}$.
Complete step by step answer:
In the lower classes, we have studied the concept called stereochemistry in the organic chemistry section which tells in detail about the arrangement of atoms in space.
We shall see in detail the stability of the conformers to deduce the required answer.
- Stereochemistry is the study of the molecules in space which tells about the arrangement of atoms in a molecule in space. Thus, this is also called 3D chemistry as the molecules are studied in the 3-dimensional form.
- Stability of the conformer is based on the Gauche form or the anti-form of the conformer.
- generally, the Gauche form is less stable compared to the anti staggered form. But, when the given compound is showing an intramolecular hydrogen bonding or ion-dipole interaction in Gauche form then this form will be more stable than the anti staggered form.
- Here, in ethane-1,2-diol, the Gauche form is more stable due to the intramolecular hydrogen bonding present in it.
- This is shown in the diagram below:

Hence, this intramolecular hydrogen bonding causes this conformation to be more stable.
So, the correct answer is “Option B”.
Note: The Gauche form of the conformer is the one in which the larger atoms are staggered adjacent to each other whereas the anti staggered or anti conformer is the one in which the larger atoms are opposite to each other that is at the angle of ${{180}^{0}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




