
Which of the carbonium ions is most stable?
A.

B.
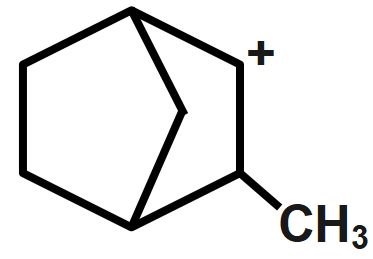
C.
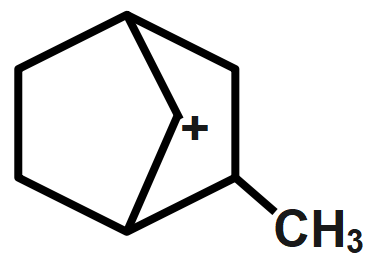
D.
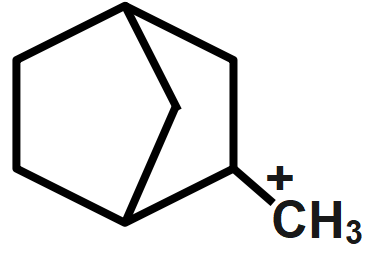




Answer
509.1k+ views
Hint: We know that the stability of carbonium ion increases as the no. of attach alkyl group on the carbonium carbon atom increases. The carbonium ion and how its stability is affected by the electron-withdrawing and electron-donating group.
Complete answer:
As we know that on the addition of a group on the carbonium ion which increases positive charge on the carbon atom stability of that carbonium ion also increases. In the given question, we must choose the correct molecule which will have the least stable carbonium ion. The carbonium ion is the ion which consists of positive charge on the molecule. Now, as we know that there are two types of molecules that are electron-withdrawing and electron-donating groups. Electron withdrawing groups are those which can accept a pair of electrons from the adjacent atom.
Whereas electron-donating groups are those which can easily donate a pair of electrons to the adjacent atom. For example, alkyl groups. So, when the alkyl group is attached to a carbonium ion, the stability of the carbonium ion will increase because of the donation of the negative charge to the positive charge. That's why more is the alkyl group attached to the carbonium ion, more will be stability. Here, option A has positive charge bridge joining that is why it's least stable from the given compounds. Similarly, option B,C has two hyper-conjugating structures and option D has six hyper-conjugating structures which makes it most stable. Thus, the most stability order can be given as $D < C,B < A.$
Therefore, the correct answer is option D.
Note:
Remember that the stability of the carbonium ion will decrease when the electron-withdrawing group is attached because it will withdraw electrons from the carbon due to which the positive charge on the carbon will increase. Also, they count hydrogen atoms as the attached alkyl group, which will lead to the wrong answer to the question. So, always make an expanded structure by showing bonds of given molecules.
Complete answer:
As we know that on the addition of a group on the carbonium ion which increases positive charge on the carbon atom stability of that carbonium ion also increases. In the given question, we must choose the correct molecule which will have the least stable carbonium ion. The carbonium ion is the ion which consists of positive charge on the molecule. Now, as we know that there are two types of molecules that are electron-withdrawing and electron-donating groups. Electron withdrawing groups are those which can accept a pair of electrons from the adjacent atom.
Whereas electron-donating groups are those which can easily donate a pair of electrons to the adjacent atom. For example, alkyl groups. So, when the alkyl group is attached to a carbonium ion, the stability of the carbonium ion will increase because of the donation of the negative charge to the positive charge. That's why more is the alkyl group attached to the carbonium ion, more will be stability. Here, option A has positive charge bridge joining that is why it's least stable from the given compounds. Similarly, option B,C has two hyper-conjugating structures and option D has six hyper-conjugating structures which makes it most stable. Thus, the most stability order can be given as $D < C,B < A.$
Therefore, the correct answer is option D.
Note:
Remember that the stability of the carbonium ion will decrease when the electron-withdrawing group is attached because it will withdraw electrons from the carbon due to which the positive charge on the carbon will increase. Also, they count hydrogen atoms as the attached alkyl group, which will lead to the wrong answer to the question. So, always make an expanded structure by showing bonds of given molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




