
Which of the following are isomers?
A) ethane and propane
B) ethane and ethene
C) ethane and ethyne
D) butane and isobutane
Answer
576k+ views
Hint: These are the examples of structural isomers. One of the compounds has a molecular formula as \[C{H_3}C{H_2}C{H_2}C{H_3}\]. One compound is branched, while the other one is unbranched.
Complete answer:
Butane and isobutane are isomers. To be precise, these are the examples of structural isomers.
Now, come to the point of what this isomer is. Isomers are the molecules, which have identical molecular formulas, which means they have the same number of atoms for each element in a compound. But the arrangements of the atoms in space are different from each other. The isomers do not necessarily exhibit the similarity in chemical or physical properties.
The isomer is of two types. One is the structural or constitutional isomer and the other is the stereoisomer or spatial isomer.
Here, butane and isobutane are structural isomers. In structural isomerism, molecules have the same number of atoms of each element but the bonds between the atoms differ.
The structure of butane and isobutane are shown below:
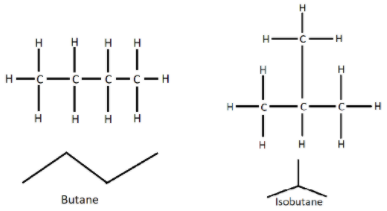
So, The correct answer is (D).
Additional Information: The main difference between butane and isobutane is that isobutane is the structural isomer of butane. Butane can convert into isobutane through the isobutane production process named isomerization. Isobutane also is a flammable hydrocarbon gas, which can be liquified through pressurization. But isobutane has different physical properties. For example, isobutane is a colorless gas with a petrol odour.
Note:
So, we can see that the molecular formula of butane is \[C{H_3}C{H_2}C{H_2}C{H_3}\]. It is an unbranched alkane molecule having four carbon atoms. On the other hand, isobutane or i-butane has the molecular formula of \[HC{\left( {C{H_3}} \right)_3}\], which is the simplest alkane with tertiary carbon, which means it has a branched structure. So, it is a structural isomer of butane.
Complete answer:
Butane and isobutane are isomers. To be precise, these are the examples of structural isomers.
Now, come to the point of what this isomer is. Isomers are the molecules, which have identical molecular formulas, which means they have the same number of atoms for each element in a compound. But the arrangements of the atoms in space are different from each other. The isomers do not necessarily exhibit the similarity in chemical or physical properties.
The isomer is of two types. One is the structural or constitutional isomer and the other is the stereoisomer or spatial isomer.
Here, butane and isobutane are structural isomers. In structural isomerism, molecules have the same number of atoms of each element but the bonds between the atoms differ.
The structure of butane and isobutane are shown below:

So, The correct answer is (D).
Additional Information: The main difference between butane and isobutane is that isobutane is the structural isomer of butane. Butane can convert into isobutane through the isobutane production process named isomerization. Isobutane also is a flammable hydrocarbon gas, which can be liquified through pressurization. But isobutane has different physical properties. For example, isobutane is a colorless gas with a petrol odour.
Note:
So, we can see that the molecular formula of butane is \[C{H_3}C{H_2}C{H_2}C{H_3}\]. It is an unbranched alkane molecule having four carbon atoms. On the other hand, isobutane or i-butane has the molecular formula of \[HC{\left( {C{H_3}} \right)_3}\], which is the simplest alkane with tertiary carbon, which means it has a branched structure. So, it is a structural isomer of butane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




