
Which of the following bond orders for bond angles is correct?
A. $B{{F}_{3}}$ < $N{{F}_{3}}$ < $Cl{{F}_{3}}$ < $P{{F}_{3}}$
B. $B{{F}_{3}}$ < $N{{F}_{3}}$ <$P{{F}_{3}}$ < $Cl{{F}_{3}}$
C. $Cl{{F}_{3}}$ < $P{{F}_{3}}$ < $N{{F}_{3}}$ < $B{{F}_{3}}$
D. $B{{F}_{3}}\approx N{{F}_{3}}$ <$P{{F}_{3}}$ < $Cl{{F}_{3}}$
Answer
542.4k+ views
Hint: As we know that bond angle is the angle formed between three atoms across at least two bonds. To find the bond angle we need to know the hybridisation of the molecules and for that we need to calculate the steric number.
Complete answer:
If we know the steric number then we can easily know the hybridisation, bond angle as well as the structure of the molecule.
- We can see from the table below the hybridisation and structure of molecule on the basis of steric number:
- The formula for calculating the steric number of uncharged molecules is the sum of valence electrons of the central atom and monovalent atom attached to the central atom divided by 2.
- We will first calculate the steric value of $B{{F}_{3}}$ :
There are 3 valence electrons present in the central atom, there are 3 monovalent atoms attached and there is no anionic or cationic charge. So, the steric number is
$=\dfrac{3+3}{2}=\dfrac{6}{2}=3$
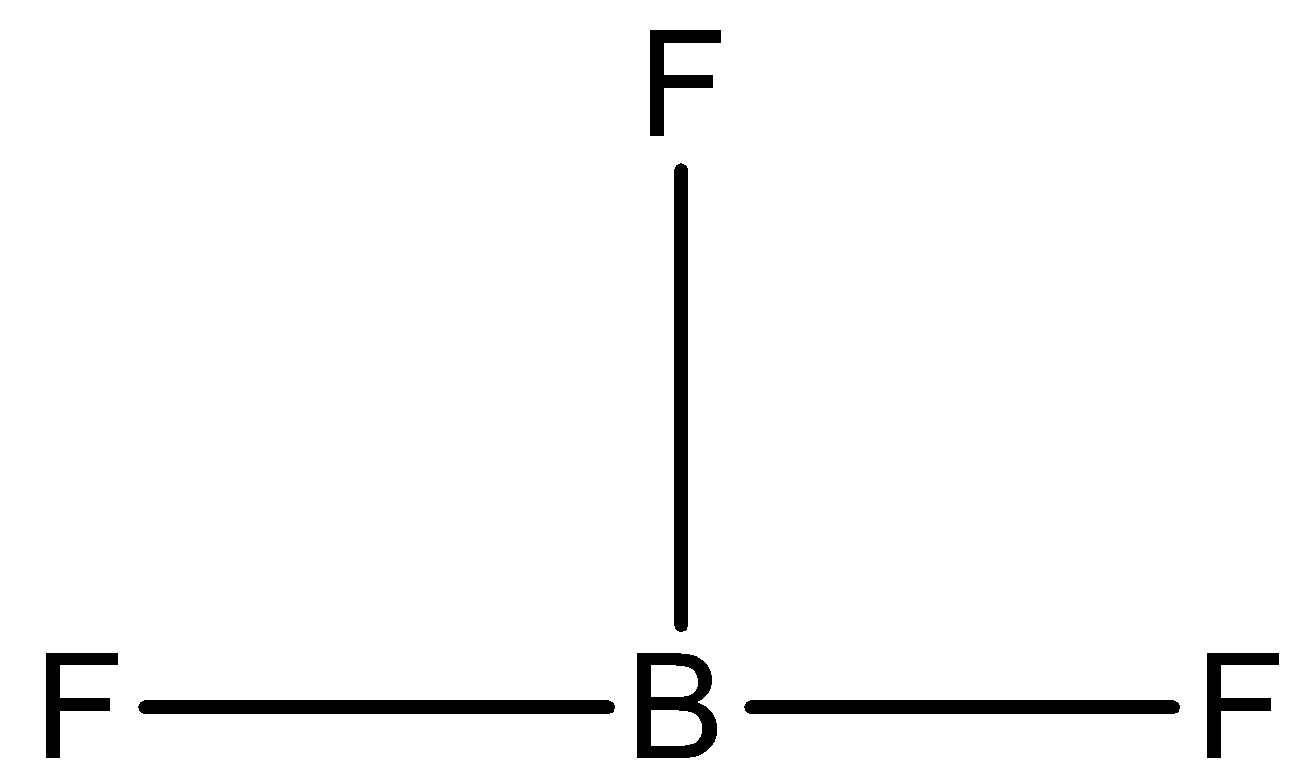
So, the hybridisation will be $s{{p}^{2}}$ and hence the bond angle will be ${{120}^{0}}$
- We will calculate the steric value of $P{{F}_{3}}$ :
There are 5 valence electrons present in the central atom, there are 3 monovalent atoms attached and there is no anionic or cationic charge. So, the steric number is
$=\dfrac{5+3}{2}=\dfrac{8}{2}=4$
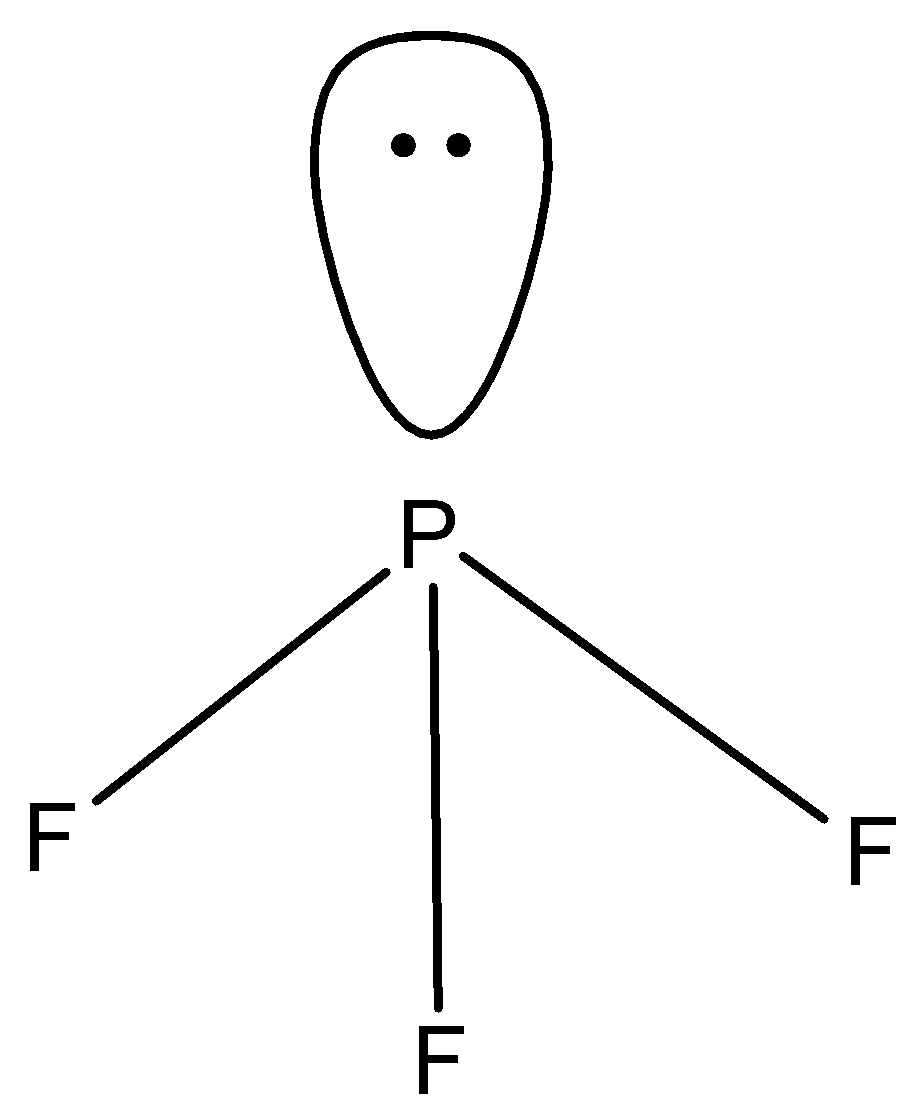
So, the hybridisation will be $s{{p}^{3}}$ and the bond angle should be ${{109}^{0}}$, but as there is one lone pair present so due to this lone pair the bond angle will decrease and will be ${{104}^{0}}$ .
- We will calculate the steric value of $N{{F}_{3}}$:
There are 5 valence electrons present in the central atom, there are 3 monovalent atoms attached and there is no anionic or cationic charge. So, the steric number is
$=\dfrac{5+3}{2}=\dfrac{8}{2}=4$
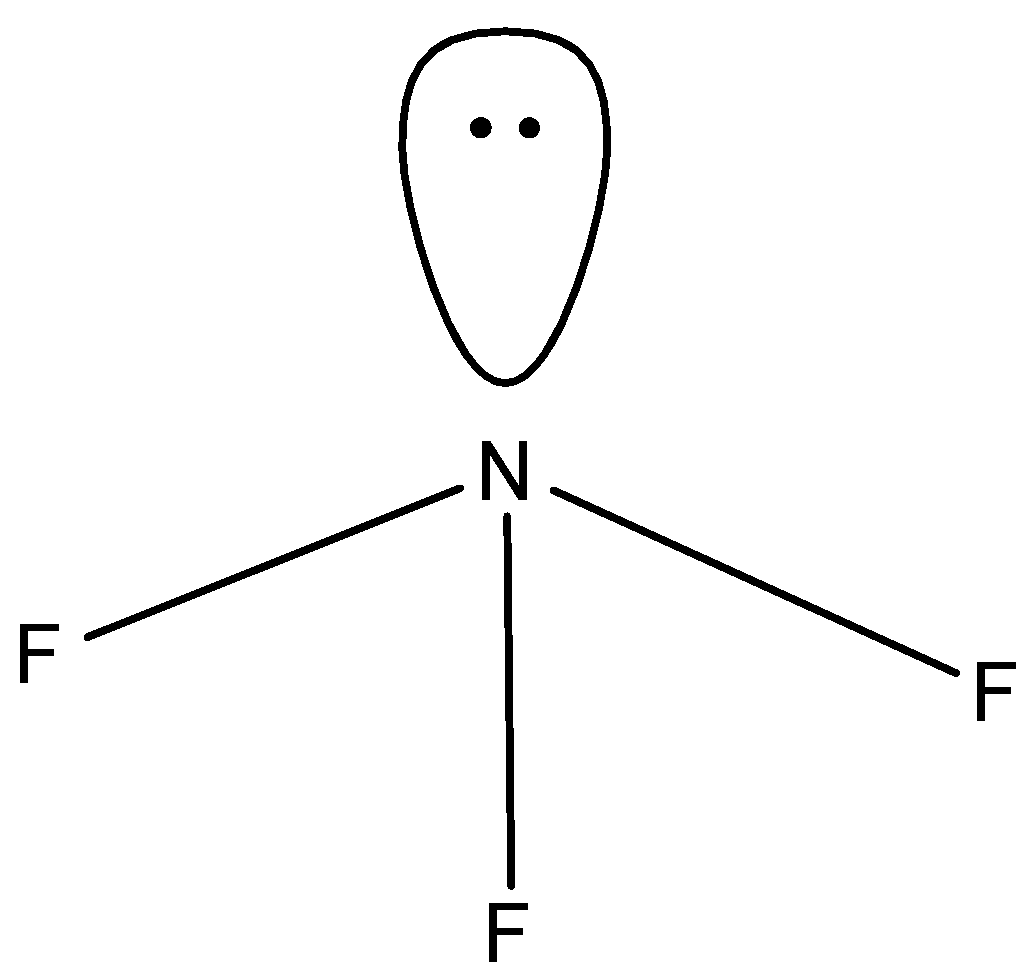
So, the hybridisation will be $s{{p}^{3}}d$ as according to VSPER theory a compound having hybridisation number 4 and 1 lone pair of electrons has a trigonal pyramidal structure and the bond angle should be ${{109.5}^{0}}$
- We will calculate the steric value of $Cl{{F}_{3}}$ :
There are 7 valence electrons present in the central atom, there are 3 monovalent atoms attached and there is no anionic or cationic charge. So, the steric number is
$\dfrac{7+3}{2}=\dfrac{10}{2}=5$
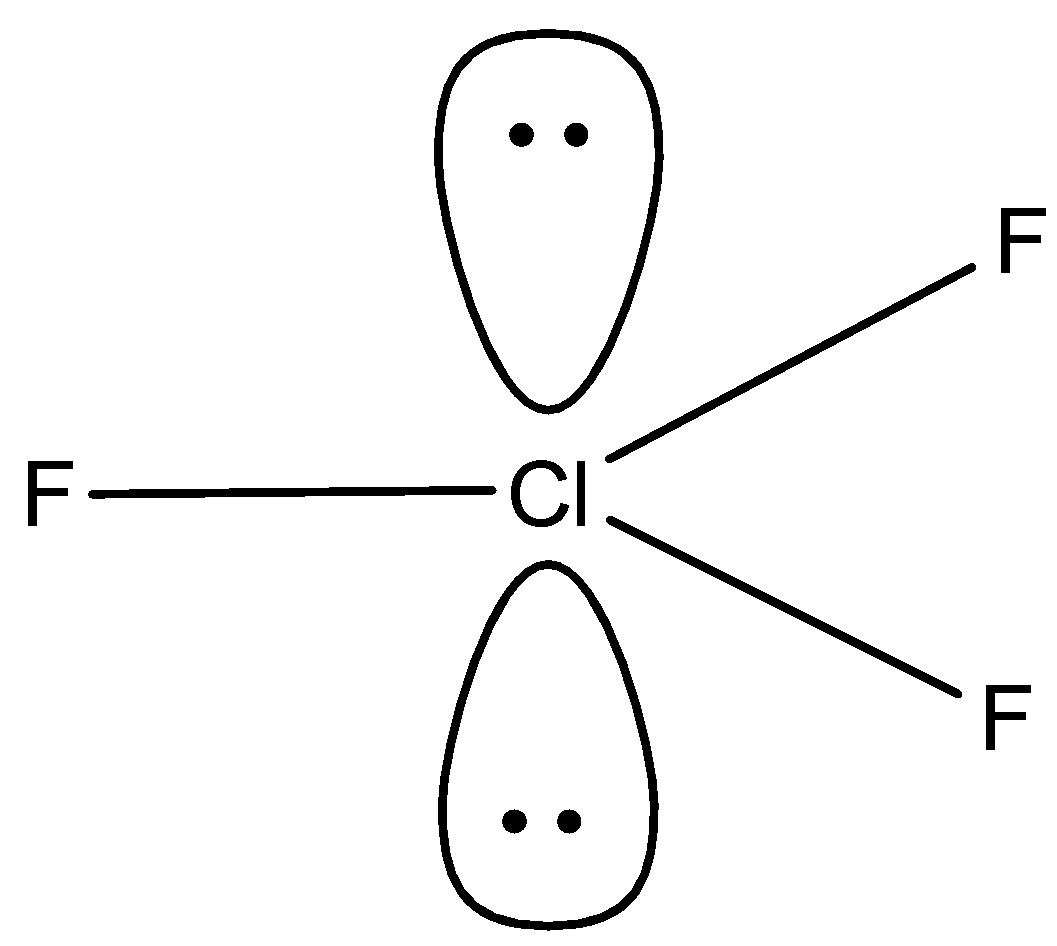
So, the hybridisation will be $s{{p}^{3}}d$ and the bond angle should be as there are two lone pairs present due to which the bond angle will decrease and will be less than ${{90}^{0}}$ .
Hence, we can conclude that the correct option is (c) that is $Cl{{F}_{3}}$ < $P{{F}_{3}}$ < $N{{F}_{3}}$ < $B{{F}_{3}}$
bond order for bond angles is correct.
Note:
- If we are being provided with any molecule that is charged then we can calculate the steric number.
- The formula for calculating steric number of anion is the sum of valence electron of central atom and monovalent atom attached to central atom and anionic charge divided by 2.
- The formula for calculating steric number of cation is sum of valence electron of central atom and monovalent atom attached to central atom and the difference of the former quantity with anionic charge divided by 2.
Complete answer:
If we know the steric number then we can easily know the hybridisation, bond angle as well as the structure of the molecule.
- We can see from the table below the hybridisation and structure of molecule on the basis of steric number:
| Steric number | Hybridisation | Structure | Bond angle |
| 2 | $sp$ | Linear | ${{180}^{0}}$ |
| 3 | $s{{p}^{2}}$ | Planar | ${{120}^{0}}$ |
| 4 | $s{{p}^{3}}$ | Tetrahedral | ${{109}^{0}}$ |
| 5 | $s{{p}^{3}}d$ | Trigonal bipyramidal | ${{90}^{0}}$ axial and ${{120}^{0}}$ equatorial |
| 6 | $s{{p}^{3}}{{d}^{2}}$ | Octahedral | ${{90}^{0}}$ |
- The formula for calculating the steric number of uncharged molecules is the sum of valence electrons of the central atom and monovalent atom attached to the central atom divided by 2.
- We will first calculate the steric value of $B{{F}_{3}}$ :
There are 3 valence electrons present in the central atom, there are 3 monovalent atoms attached and there is no anionic or cationic charge. So, the steric number is
$=\dfrac{3+3}{2}=\dfrac{6}{2}=3$

So, the hybridisation will be $s{{p}^{2}}$ and hence the bond angle will be ${{120}^{0}}$
- We will calculate the steric value of $P{{F}_{3}}$ :
There are 5 valence electrons present in the central atom, there are 3 monovalent atoms attached and there is no anionic or cationic charge. So, the steric number is
$=\dfrac{5+3}{2}=\dfrac{8}{2}=4$

So, the hybridisation will be $s{{p}^{3}}$ and the bond angle should be ${{109}^{0}}$, but as there is one lone pair present so due to this lone pair the bond angle will decrease and will be ${{104}^{0}}$ .
- We will calculate the steric value of $N{{F}_{3}}$:
There are 5 valence electrons present in the central atom, there are 3 monovalent atoms attached and there is no anionic or cationic charge. So, the steric number is
$=\dfrac{5+3}{2}=\dfrac{8}{2}=4$

So, the hybridisation will be $s{{p}^{3}}d$ as according to VSPER theory a compound having hybridisation number 4 and 1 lone pair of electrons has a trigonal pyramidal structure and the bond angle should be ${{109.5}^{0}}$
- We will calculate the steric value of $Cl{{F}_{3}}$ :
There are 7 valence electrons present in the central atom, there are 3 monovalent atoms attached and there is no anionic or cationic charge. So, the steric number is
$\dfrac{7+3}{2}=\dfrac{10}{2}=5$

So, the hybridisation will be $s{{p}^{3}}d$ and the bond angle should be as there are two lone pairs present due to which the bond angle will decrease and will be less than ${{90}^{0}}$ .
Hence, we can conclude that the correct option is (c) that is $Cl{{F}_{3}}$ < $P{{F}_{3}}$ < $N{{F}_{3}}$ < $B{{F}_{3}}$
bond order for bond angles is correct.
Note:
- If we are being provided with any molecule that is charged then we can calculate the steric number.
- The formula for calculating steric number of anion is the sum of valence electron of central atom and monovalent atom attached to central atom and anionic charge divided by 2.
- The formula for calculating steric number of cation is sum of valence electron of central atom and monovalent atom attached to central atom and the difference of the former quantity with anionic charge divided by 2.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

What is the difference between biodegradable and nonbiodegradable class 11 biology CBSE

Proton was discovered by A Thomson B Rutherford C Chadwick class 11 chemistry CBSE




