
Which of the following cannot be prepared by Sandmeyer's reaction?
(A) Chlorobenzene
(B) Bromobenzene
(C) Iodobenzene
(D) Fluorobenzene
Answer
522.3k+ views
Hint: As we know that the Sandmeyer’s reaction is a chemical reaction in organic chemistry which is used in the synthesis of aryl halides from aryl diazonium salts by using copper salts as reagents or catalysts. This reaction is an example of radical-nucleophilic aromatic substitution. So here we have to tell which compound cannot be prepared by Sandmeyer's reaction.
Complete answer:
Let us first discuss about Sandmeyer's reaction as follows:-
-Sandmeyer's reaction: It is a chemical reaction in organic chemistry which is used in the synthesis of aryl halides from aryl diazonium salts by using copper salts as reagents or catalysts. This reaction is useful as an amino group on an aromatic ring can be substituted with different groups. During this reaction, the amino group which is attached to an aromatic ring is converted into diazonium salt that is ultimately transformed into various functional groups as desired.
-The reaction takes place as follows:-
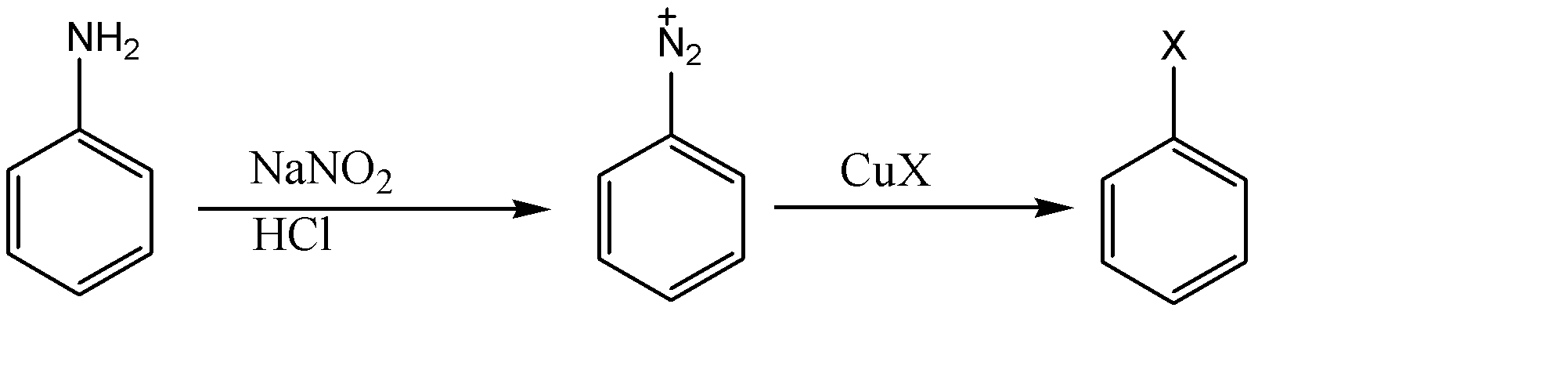
As we can see, sodium nitrate is used with hydrochloric acid to convert amino groups on benzene rings to diazonium salt. Now we can bring any substituent at that place using various reagents. When copper salts of halide are used as reagent in the reaction, then it is referred to as Sandmeyer's reaction.
-Generally copper salt of chlorine and bromine are available and hence used for the production of chlorobenzene and bromobenzene whereas iodobenzene is produced using potassium iodide (KI) instead of copper salt and therefore it cannot be produced through Sandmeyer's reaction.
-For the formation of fluorobenzene, diazonium salt is made to react with fluoroboric acid ($HB{{F}_{4}}$) and is heated at high temperature. This reaction is known as the Balz Schiemann reaction.
From the above data we can conclude that (C) Iodobenzene and (D) Fluorobenzene cannot be prepared by Sandmeyer's reaction.
Note:
-Remember that $NaN{{O}_{2}}$ reacts with HCl to form $HN{{O}_{2}}$ which acts as a intermediate in the formation of diazonium salt and this reaction takes place at 0 to 5$^{\circ }C$ temperature.
-Sandmeyer's reaction is also used to produce benzonitriles through the process of cyanation.
Complete answer:
Let us first discuss about Sandmeyer's reaction as follows:-
-Sandmeyer's reaction: It is a chemical reaction in organic chemistry which is used in the synthesis of aryl halides from aryl diazonium salts by using copper salts as reagents or catalysts. This reaction is useful as an amino group on an aromatic ring can be substituted with different groups. During this reaction, the amino group which is attached to an aromatic ring is converted into diazonium salt that is ultimately transformed into various functional groups as desired.
-The reaction takes place as follows:-

As we can see, sodium nitrate is used with hydrochloric acid to convert amino groups on benzene rings to diazonium salt. Now we can bring any substituent at that place using various reagents. When copper salts of halide are used as reagent in the reaction, then it is referred to as Sandmeyer's reaction.
-Generally copper salt of chlorine and bromine are available and hence used for the production of chlorobenzene and bromobenzene whereas iodobenzene is produced using potassium iodide (KI) instead of copper salt and therefore it cannot be produced through Sandmeyer's reaction.
-For the formation of fluorobenzene, diazonium salt is made to react with fluoroboric acid ($HB{{F}_{4}}$) and is heated at high temperature. This reaction is known as the Balz Schiemann reaction.
From the above data we can conclude that (C) Iodobenzene and (D) Fluorobenzene cannot be prepared by Sandmeyer's reaction.
Note:
-Remember that $NaN{{O}_{2}}$ reacts with HCl to form $HN{{O}_{2}}$ which acts as a intermediate in the formation of diazonium salt and this reaction takes place at 0 to 5$^{\circ }C$ temperature.
-Sandmeyer's reaction is also used to produce benzonitriles through the process of cyanation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




