
Which of the following cannot be used as a nitrating agent in Electrophilic Aromatic Substitution of benzene?
(A)- ${{N}_{2}}{{O}_{5}}/MeCN$
(B)- ${{C}_{2}}{{H}_{5}}ON{{O}_{2}}$
(C)- ${{C}_{2}}{{H}_{5}}N{{O}_{2}}$
(D)- $N{{O}_{2}},S{{O}_{3}}$
Answer
578.4k+ views
Hint: An electrophilic aromatic substitution reaction is a reaction in which C-H bond is broken and a new carbon bond with electrophilic atom such as Cl Br, N is formed. Unlike alkenes, benzenes and other aromatic compounds do not generally undergo addition reactions. Addition of electrophiles in such compounds occurs through substitution reaction.
Complete step by step solution:
-Electrophilic aromatic substitution of benzene or other compounds are greatly facilitated by the addition of a Lewis acid as catalyst.
-The key pattern of electrophilic aromatic substitution reaction is given as-
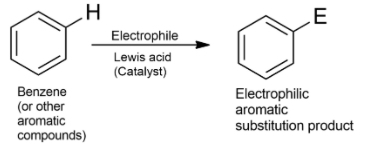
-Nitration is the replacement or substitution of H with a nitro $(N{{O}_{2}})$ group using nitrating agents.
-Let us now analyse the compounds given to us options to identify which compound can and cannot be used as nitrating agents.
-The anhydride form of nitric acid is dinitrogen pentoxide $({{N}_{2}}{{O}_{5}})$which can be prepared by carefully dehydrating nitric acid with diphosphorus pentoxide or by oxidizing nitrogen dioxide with ozone. Dinitrogen pentoxide is a highly unstable and mildly explosive compound. It is used as a nitrating agent in modern synthetic organic chemistry as a mixture with $HN{{O}_{3}}$.
-Ethyl nitrate is a highly flammable, reactive, and dangerous fire compound. It is a colourless liquid with a sweet taste and pleasant odour. It is used as a nitrating agent for aromatic compounds and also in the making of drugs, perfumes and dyes.
-Ethyl nitrite is an alkyl nitrite which may be prepared from ethanol. This is also known as nitre or sweet spirit which is used as a nitrating agent in the reaction with butanone yielding the dimethylglyoxime end.
-Benzene reacts with nitric acid at 323 K- 333 K in the presence of sulphuric acid forming $N{{O}_{2}},S{{O}_{3}}$ ions which cause nitration of benzene yielding nitrobenzene.
Hence, the incorrect combination in the option to be a nitrating agent for benzene aromatic substitutions is option A.
Note: Resonance involved in the benzene ring makes the delocalized electron to relocate effectively over the carbon atoms in the benzene ring. It partially stabilizes the arenium ion too making benzene more prone to electrophilic substitution reactions.
Complete step by step solution:
-Electrophilic aromatic substitution of benzene or other compounds are greatly facilitated by the addition of a Lewis acid as catalyst.
-The key pattern of electrophilic aromatic substitution reaction is given as-

-Nitration is the replacement or substitution of H with a nitro $(N{{O}_{2}})$ group using nitrating agents.
-Let us now analyse the compounds given to us options to identify which compound can and cannot be used as nitrating agents.
-The anhydride form of nitric acid is dinitrogen pentoxide $({{N}_{2}}{{O}_{5}})$which can be prepared by carefully dehydrating nitric acid with diphosphorus pentoxide or by oxidizing nitrogen dioxide with ozone. Dinitrogen pentoxide is a highly unstable and mildly explosive compound. It is used as a nitrating agent in modern synthetic organic chemistry as a mixture with $HN{{O}_{3}}$.
-Ethyl nitrate is a highly flammable, reactive, and dangerous fire compound. It is a colourless liquid with a sweet taste and pleasant odour. It is used as a nitrating agent for aromatic compounds and also in the making of drugs, perfumes and dyes.
-Ethyl nitrite is an alkyl nitrite which may be prepared from ethanol. This is also known as nitre or sweet spirit which is used as a nitrating agent in the reaction with butanone yielding the dimethylglyoxime end.
-Benzene reacts with nitric acid at 323 K- 333 K in the presence of sulphuric acid forming $N{{O}_{2}},S{{O}_{3}}$ ions which cause nitration of benzene yielding nitrobenzene.
Hence, the incorrect combination in the option to be a nitrating agent for benzene aromatic substitutions is option A.
Note: Resonance involved in the benzene ring makes the delocalized electron to relocate effectively over the carbon atoms in the benzene ring. It partially stabilizes the arenium ion too making benzene more prone to electrophilic substitution reactions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




