
Which of the following carbanions is stabilized?
A.
B.
C.
D.




Answer
566.7k+ views
Hint: A carbanion is an organic ion with an unshared pair of electrons and a negative charge on the central carbon atom.When a group Y, less electronegative than carbon is attached to it, the bond $\left( {C - Y} \right)$in an organic molecule breaks heterolytically. The carbon takes away the bonding electron pair and acquires a negative charge while a positive ion ${Y^ + }$ is produced
Complete step by step answer:
Now we will discuss the given options one by one.
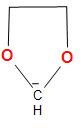
A.The structure of the carbanion given in the first option is shown below:
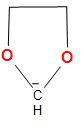
In the above structure, we can see that two oxygen atom and two methyl groups are attached to the carbanion. As we know that oxygen contains two lone pairs of electrons and here the two oxygen atom will have four pairs of lone pair of electrons and the two methyl groups $\left( { + I} \right)$, which will increase the electron density on the negatively charged carbon atom or carbanion and thus, decreases the stability. Therefore, the given carbanion is less stabilized.
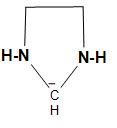
B.The structure of the carbanion given in the second option is shown below:
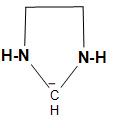
In the above structure, we can see that two ammonia and two methyl groups are attached to the carbanion. As we know that ammonia contains one pair of electrons and here two ammonia will have two lone pairs and the two methyl groups, which will increase the electron density on the negatively charged carbon atom or carbanion and thus, decreases the stability. Therefore, the given carbanion is less stabilized.
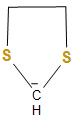
C.The structure of the carbanion given in the third option is shown below:
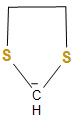
In the above structure, we can see that two sulphur atoms and two methyl groups are attached to the carbanion. As we know that one sulphur atom contains one lone pair of electrons and here the two sulphur atom will have two lone pairs and the two methyl groups, which will increase the electron density on the negatively charged carbon atom or carbanion and thus, decreases the stability. Therefore, the given carbanion is less stabilized.
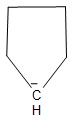
D.The structure of the carbanion given in the fourth option is shown below:
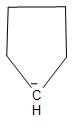
In the above structure, we can see that there is no substituent but two methyl groups are attached to the carbanion. Due to the inductive effect, the two methyl groups will increase the electron density on the carbanion and thus, decrease the stability. Thus, the given carbanion will be less stabilized.
All the carbanions are less stabilized but the carbanion containing only two methyls will be stabilized in comparison to the other carbanions.
So, the correct answer is Option D .
Note: The negatively charged carbon atom in carbanion is $s{p^3}$ hybridized giving a tetrahedral structure.Since carbanions are electron-rich species, they behave as nucleophiles and they combine with electrophiles to form neutral molecules and also give many additions and substitution products.
Complete step by step answer:
Now we will discuss the given options one by one.
A.The structure of the carbanion given in the first option is shown below:

In the above structure, we can see that two oxygen atom and two methyl groups are attached to the carbanion. As we know that oxygen contains two lone pairs of electrons and here the two oxygen atom will have four pairs of lone pair of electrons and the two methyl groups $\left( { + I} \right)$, which will increase the electron density on the negatively charged carbon atom or carbanion and thus, decreases the stability. Therefore, the given carbanion is less stabilized.
B.The structure of the carbanion given in the second option is shown below:

In the above structure, we can see that two ammonia and two methyl groups are attached to the carbanion. As we know that ammonia contains one pair of electrons and here two ammonia will have two lone pairs and the two methyl groups, which will increase the electron density on the negatively charged carbon atom or carbanion and thus, decreases the stability. Therefore, the given carbanion is less stabilized.
C.The structure of the carbanion given in the third option is shown below:

In the above structure, we can see that two sulphur atoms and two methyl groups are attached to the carbanion. As we know that one sulphur atom contains one lone pair of electrons and here the two sulphur atom will have two lone pairs and the two methyl groups, which will increase the electron density on the negatively charged carbon atom or carbanion and thus, decreases the stability. Therefore, the given carbanion is less stabilized.
D.The structure of the carbanion given in the fourth option is shown below:

In the above structure, we can see that there is no substituent but two methyl groups are attached to the carbanion. Due to the inductive effect, the two methyl groups will increase the electron density on the carbanion and thus, decrease the stability. Thus, the given carbanion will be less stabilized.
All the carbanions are less stabilized but the carbanion containing only two methyls will be stabilized in comparison to the other carbanions.
So, the correct answer is Option D .
Note: The negatively charged carbon atom in carbanion is $s{p^3}$ hybridized giving a tetrahedral structure.Since carbanions are electron-rich species, they behave as nucleophiles and they combine with electrophiles to form neutral molecules and also give many additions and substitution products.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




