
Which of the following carbocation would expect to rearrange?
(A)

(B)
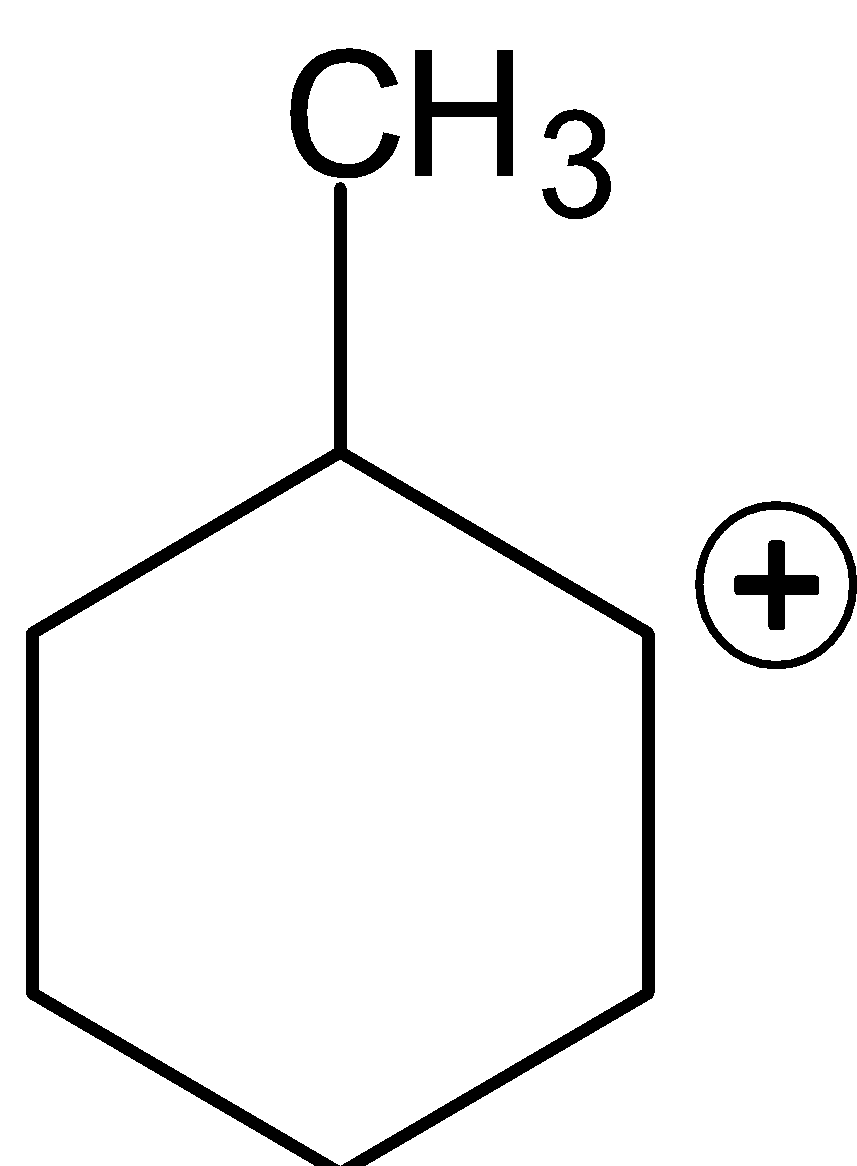
(C)
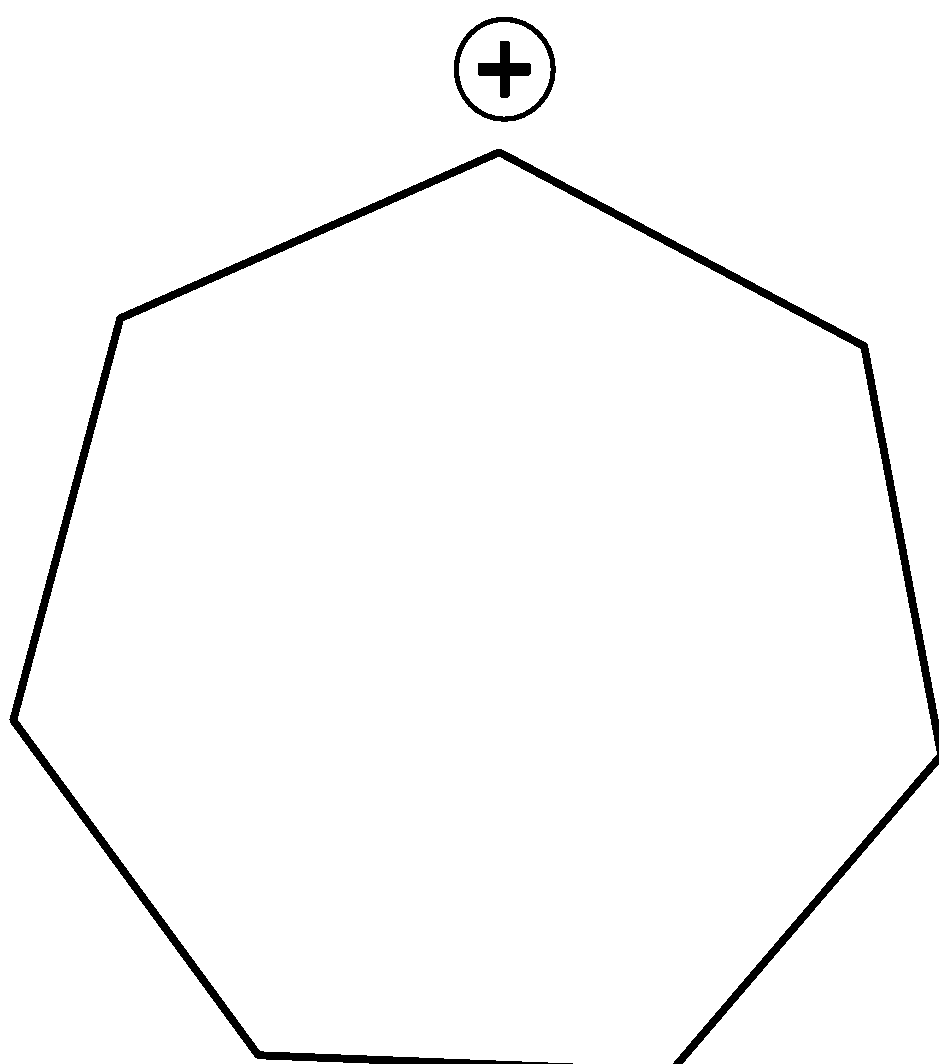



Answer
573.6k+ views
Hint: Firstly, we should be aware of carbocation and we should understand when the rearrangement in carbocation and how it takes place. Knowing these will help us answer questions within no time.
Complete step by step answer:
In a heterolytic fission of a C-X bond in a molecule of an organic compound. Here if X is more electronegative than the carbon atom, the X takes away the bonding pair of electrons. Two ions are obtained as a product. An ion bearing positive charge and negative charge the positively charged species is known as carbocation and the negatively charged species is known as carbanion..
In a carbocation the presence of an electron releasing group such as an alkyl group adjacent to the carbon that is bearing positive charge increases the stability of the carbocation. The tertiary carbocation is more stable than the secondary carbocation followed by primary carbocation.
The carbocation undergoes rearrangement to give a more stable carbocation.
For example: A primary carbocation undergoes rearrangement in order to give more stable secondary carbocation.
Option A is a secondary carbocation. If we perform rearrangement in this carbocation it again gives secondary carbocation that is it will remain as secondary carbocation. Therefore, this option is the wrong answer.
Option B is also a secondary carbocation. If we perform rearrangement in this carbocation it will give more stable tertiary carbocation. Therefore, we can say that this carbocation undergoes rearrangement. Thus, this is the correct answer.
Option C is a secondary carbocation. If we perform rearrangement in this carbocation it again gives secondary carbocation that is it will remain as secondary carbocation. Therefore, this option is the wrong answer.
Thus, option B is the correct answer.
Note: The carbocation undergoes rearrangement to form more stable carbocation. Even after performing rearrangement if it does not arrive at the more stable carbocation just like incase of option A and option C then, we can say that there is no rearrangement taking place.
Complete step by step answer:
In a heterolytic fission of a C-X bond in a molecule of an organic compound. Here if X is more electronegative than the carbon atom, the X takes away the bonding pair of electrons. Two ions are obtained as a product. An ion bearing positive charge and negative charge the positively charged species is known as carbocation and the negatively charged species is known as carbanion..
In a carbocation the presence of an electron releasing group such as an alkyl group adjacent to the carbon that is bearing positive charge increases the stability of the carbocation. The tertiary carbocation is more stable than the secondary carbocation followed by primary carbocation.
The carbocation undergoes rearrangement to give a more stable carbocation.
For example: A primary carbocation undergoes rearrangement in order to give more stable secondary carbocation.
Option A is a secondary carbocation. If we perform rearrangement in this carbocation it again gives secondary carbocation that is it will remain as secondary carbocation. Therefore, this option is the wrong answer.
Option B is also a secondary carbocation. If we perform rearrangement in this carbocation it will give more stable tertiary carbocation. Therefore, we can say that this carbocation undergoes rearrangement. Thus, this is the correct answer.
Option C is a secondary carbocation. If we perform rearrangement in this carbocation it again gives secondary carbocation that is it will remain as secondary carbocation. Therefore, this option is the wrong answer.
Thus, option B is the correct answer.
Note: The carbocation undergoes rearrangement to form more stable carbocation. Even after performing rearrangement if it does not arrive at the more stable carbocation just like incase of option A and option C then, we can say that there is no rearrangement taking place.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




