
Which of the following compounds contain active methylene groups?
(This question has multiple correct options)

Answer
555.3k+ views
Hint: The question arises what are active methylene groups. So, as the name suggests that active methylene, it means it is a methylene group $ - C{H_2} - $ and it should have an electron withdrawing group just in the neighborhood such that the hydrogen attached with it is active. By means active I mean that any base can abstract it.
Complete step-by-step answer:
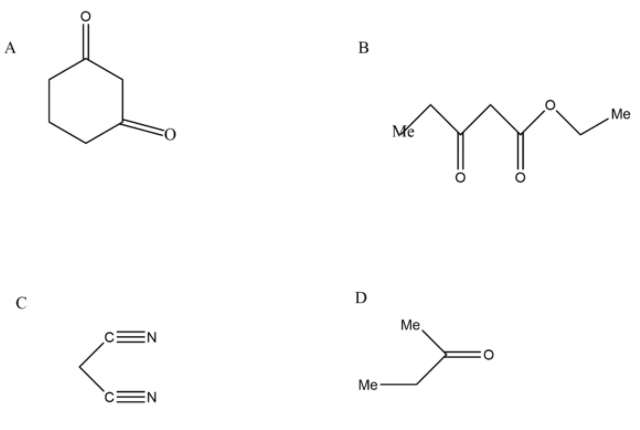
In the first option we have a cyclohexane ring in which there are two carbonyl groups which are having electron withdrawing nature, thus the methylene in between them is called an active methylene group. Its hydrogen can be abstract with the help of a base very easily therefore it is having an active methylene group and can be our answer.
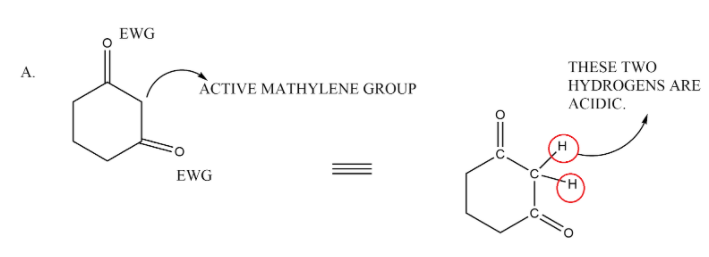
In the second option we have a straight line compound in which there are also two electron withdrawing groups that are carbonyls which withdraw the electrons from the methylene group and make its hydrogen very acidic. It can also be our answer.
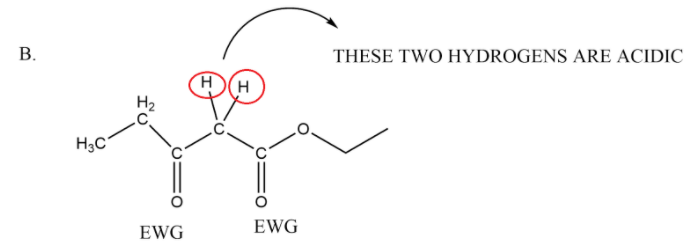
In the third option we have cyanides which are two in number and connected with a methylene group, these cyanides are also electron withdrawing in nature so they will withdraw electron density and make the proton on methylene group acidic. Thus it is also having an active methylene group.
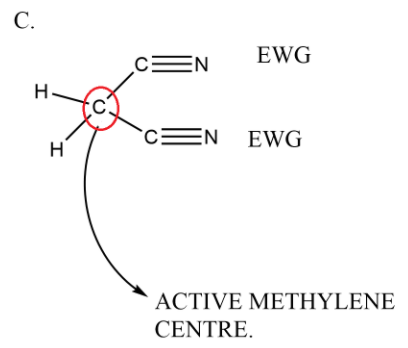
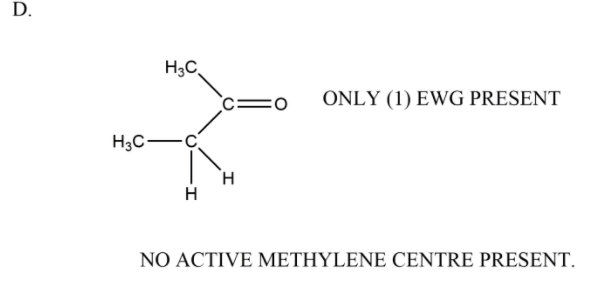
In the last option we have only one carbonyl which is yes an electron withdrawing group but it is only one in number so the methylene group adjacent to it is not active in nature.
Hence the correct answer are option ‘A’,’B’ and ‘C’
Note: Keep in mind that when you are seeing or finding an active methylene site there should be an electron withdrawing group by side of it, which withdraws the electron density and makes the proton of methylene group acidic. After withdrawing of that proton the carbanion formed is stable and can form enolate further.
Complete step-by-step answer:
In the first option we have a cyclohexane ring in which there are two carbonyl groups which are having electron withdrawing nature, thus the methylene in between them is called an active methylene group. Its hydrogen can be abstract with the help of a base very easily therefore it is having an active methylene group and can be our answer.

In the second option we have a straight line compound in which there are also two electron withdrawing groups that are carbonyls which withdraw the electrons from the methylene group and make its hydrogen very acidic. It can also be our answer.

In the third option we have cyanides which are two in number and connected with a methylene group, these cyanides are also electron withdrawing in nature so they will withdraw electron density and make the proton on methylene group acidic. Thus it is also having an active methylene group.

In the last option we have only one carbonyl which is yes an electron withdrawing group but it is only one in number so the methylene group adjacent to it is not active in nature.

Hence the correct answer are option ‘A’,’B’ and ‘C’
Note: Keep in mind that when you are seeing or finding an active methylene site there should be an electron withdrawing group by side of it, which withdraws the electron density and makes the proton of methylene group acidic. After withdrawing of that proton the carbanion formed is stable and can form enolate further.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




