
Which of the following compounds is aromatic alcohol?
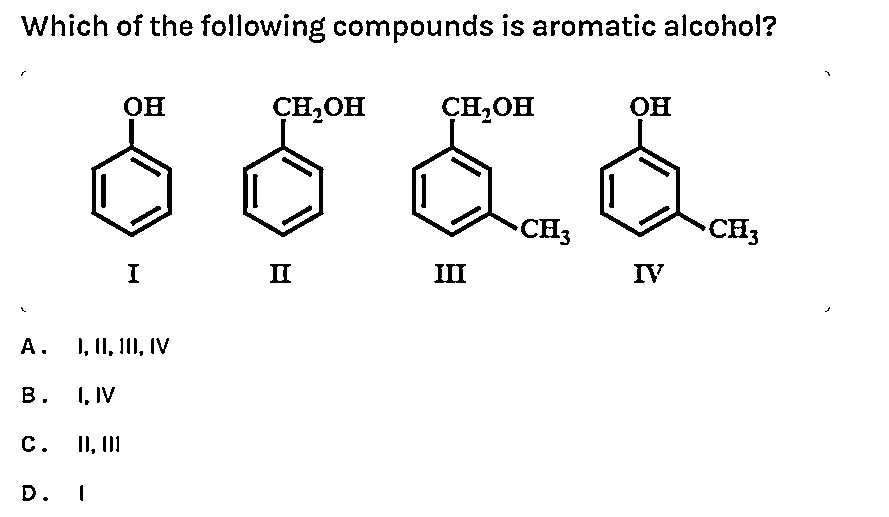
A. I,II,III,IV
B. I, IV
C. II,III
D. I

Answer
604.5k+ views
Hint: We should know that aromatic alcohols or aryl-alcohols are a class of chemical compounds containing a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group.
Complete step-by-step answer:
In this question, we have to check whether above represented figures are aromatic alcohols or not. To check we should know about one important thing about aromatic alcohols. We should know that the aromatic alcohols are those compounds in which the hydroxyl group is not directly attached to the benzene ring but is linked to a carbon atom situated in a side-chain. In short, we will search for that figure in which the hydroxyl group is linked to $s{{p}^{3}}$ hybridised carbon.
We should answer this question by observing each figure. We will check each figure and then we will answer this question.
So, by looking at figure one, we observe that this structure is of phenol. We should know that phenols have unique properties and are not classified as alcohols. They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. Phenol is more acidic than acyclic alcohols because the phenoxide ion is more stable than the alkoxide ion. So, now we can say that it is not an aromatic alcohol.
If we look at figure two then we will observe that it is a structure of benzyl alcohol. The IUPAC name of this compound is phenyl methanol. This compound consists of a hydroxyl group attached to a methyl group, which is in turn attached to an aromatic ring. This structure satisfies the requirements to be aromatic alcohol. So, it is an aromatic alcohol.
If we take option C, in this structure we observe that the hydroxyl group is attached to \[s{{p}^{3}}\] hybridized carbon. Or we can say that a hydroxyl group attached to a methyl group, which is in turn attached to an aromatic ring. This structure satisfies the requirements to be aromatic alcohol. So, it is an aromatic alcohol.
In option D, we observe that it is a derivative of phenol. In this, the hydroxyl group is attached directly to the benzene ring. So, it cannot be considered as aromatic alcohol.
So, from the above discussion we can now conclude that structures represented in figure second and third are aromatic alcohols but structures in figure first and fourth are not aromatic alcohols. So, from this we can now say that option C is correct.
Note: We should note that phenols are the organic compounds containing a benzene ring bonded to a hydroxyl group. They are also known as carbolic acids. Phenols react with active metals like sodium, potassium to form phenoxide. This reaction of phenol with metals indicates its acidic nature. Phenols react with aqueous sodium hydroxide too to produce phenoxide ions. This indicates that the acidity of phenols is higher in comparison to the alcohols and water molecules.
Complete step-by-step answer:
In this question, we have to check whether above represented figures are aromatic alcohols or not. To check we should know about one important thing about aromatic alcohols. We should know that the aromatic alcohols are those compounds in which the hydroxyl group is not directly attached to the benzene ring but is linked to a carbon atom situated in a side-chain. In short, we will search for that figure in which the hydroxyl group is linked to $s{{p}^{3}}$ hybridised carbon.
We should answer this question by observing each figure. We will check each figure and then we will answer this question.
So, by looking at figure one, we observe that this structure is of phenol. We should know that phenols have unique properties and are not classified as alcohols. They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. Phenol is more acidic than acyclic alcohols because the phenoxide ion is more stable than the alkoxide ion. So, now we can say that it is not an aromatic alcohol.
If we look at figure two then we will observe that it is a structure of benzyl alcohol. The IUPAC name of this compound is phenyl methanol. This compound consists of a hydroxyl group attached to a methyl group, which is in turn attached to an aromatic ring. This structure satisfies the requirements to be aromatic alcohol. So, it is an aromatic alcohol.
If we take option C, in this structure we observe that the hydroxyl group is attached to \[s{{p}^{3}}\] hybridized carbon. Or we can say that a hydroxyl group attached to a methyl group, which is in turn attached to an aromatic ring. This structure satisfies the requirements to be aromatic alcohol. So, it is an aromatic alcohol.
In option D, we observe that it is a derivative of phenol. In this, the hydroxyl group is attached directly to the benzene ring. So, it cannot be considered as aromatic alcohol.
So, from the above discussion we can now conclude that structures represented in figure second and third are aromatic alcohols but structures in figure first and fourth are not aromatic alcohols. So, from this we can now say that option C is correct.
Note: We should note that phenols are the organic compounds containing a benzene ring bonded to a hydroxyl group. They are also known as carbolic acids. Phenols react with active metals like sodium, potassium to form phenoxide. This reaction of phenol with metals indicates its acidic nature. Phenols react with aqueous sodium hydroxide too to produce phenoxide ions. This indicates that the acidity of phenols is higher in comparison to the alcohols and water molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




