
Which of the following compounds will show stereoisomerism?
A.$1 - {\text{phenylethanamine}}$
B.${\text{phenyl chloroethanoate}}$
C.${\text{3 - chloro - 3 - ethynylpenta - 1,4 - diyne}}$
D.$3 - {\text{methylbutanoic acid}}$
Answer
578.1k+ views
Hint: The isomers in which atoms are bonded to each other in the same order but that differ in the arrangement of these atoms in the space are called stereo-isomers and the phenomenon is called stereo-isomerism. The main condition for stereoisomerism is that the compound should have a chiral center or geometrical center.
Complete step by step answer:
We have to find the compound which has stereoisomerism. So for optical isomerism, we will check if there is a chiral carbon in the given compounds or not. Chiral carbon is the carbon in which all four valencies of carbon are satisfied by different elements. Geometric isomerism is generally shown by unsaturated carbons or cyclic compounds. So we will also check whether the compound has a carbon-carbon double bond or not.
$1 - {\text{phenylethanamine}}$- Shows optical stereoisomerism as it has a chiral center. The structure of the compound is given as-
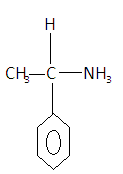
Here we can see that the carbon has all the different elements attached to it to satisfy all four valencies. So it is an optically active compound as it will rotate the directions of plane polarized light.
In ${\text{phenylchloroethanoate}}$- there is no chiral center or geometric center so it will not show stereoisomerism. Its structure is –
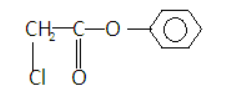
Here all the four valencies of the carbon are satisfied by two same elements and two different elements.
${\text{3 - chloro - 3 - ethynylpenta - 1,4 - diyne}}$-has no carbon-carbon double bond, it has only carbon-carbon triple bond so it will not have geometrical isomerism. Here the central carbon has three same molecules attached to it so it will not have a chiral center. Its structure is given as-
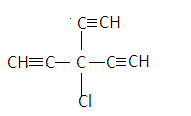
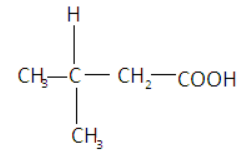
$3 - {\text{methylbutanoic acid}}$-does not show isomerism as the central atom (carbon) has $2$ same elements and $2$ different elements attached to it. So it does not have chiral carbon. Also there is no carbon-carbon double bond so there is no geometric center. Its structure is given as-
Hence option A is correct.
Note:
Stereoisomerism is of two types-
Geometrical Isomerism-In this isomerism the isomers differ in geometric arrangement of the ligands or molecules. If the arrangement of same molecules is on same side of the central atom then it is called cis-isomers. If the arrangement of the same molecules is on the opposite side of the central atom then it is called trans-isomers.
Optical Isomerism- In this isomerism, the compounds have the same formula but differ in their ability to rotate directions of the plane of polarized light. The compounds are said to be optically active and the isomers are named optical isomers.
Complete step by step answer:
We have to find the compound which has stereoisomerism. So for optical isomerism, we will check if there is a chiral carbon in the given compounds or not. Chiral carbon is the carbon in which all four valencies of carbon are satisfied by different elements. Geometric isomerism is generally shown by unsaturated carbons or cyclic compounds. So we will also check whether the compound has a carbon-carbon double bond or not.
$1 - {\text{phenylethanamine}}$- Shows optical stereoisomerism as it has a chiral center. The structure of the compound is given as-

Here we can see that the carbon has all the different elements attached to it to satisfy all four valencies. So it is an optically active compound as it will rotate the directions of plane polarized light.
In ${\text{phenylchloroethanoate}}$- there is no chiral center or geometric center so it will not show stereoisomerism. Its structure is –

Here all the four valencies of the carbon are satisfied by two same elements and two different elements.
${\text{3 - chloro - 3 - ethynylpenta - 1,4 - diyne}}$-has no carbon-carbon double bond, it has only carbon-carbon triple bond so it will not have geometrical isomerism. Here the central carbon has three same molecules attached to it so it will not have a chiral center. Its structure is given as-
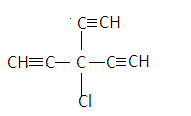
$3 - {\text{methylbutanoic acid}}$-does not show isomerism as the central atom (carbon) has $2$ same elements and $2$ different elements attached to it. So it does not have chiral carbon. Also there is no carbon-carbon double bond so there is no geometric center. Its structure is given as-

Hence option A is correct.
Note:
Stereoisomerism is of two types-
Geometrical Isomerism-In this isomerism the isomers differ in geometric arrangement of the ligands or molecules. If the arrangement of same molecules is on same side of the central atom then it is called cis-isomers. If the arrangement of the same molecules is on the opposite side of the central atom then it is called trans-isomers.
Optical Isomerism- In this isomerism, the compounds have the same formula but differ in their ability to rotate directions of the plane of polarized light. The compounds are said to be optically active and the isomers are named optical isomers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




