
Which of the following contains a double bond?
A.Carbon Dioxide
B.Chlorine
C.Ethyne
D.Water
Answer
567.3k+ views
Hint:In the given question we have to observe that all of the options are covalent compounds. Now we have the options that are Carbon dioxide, Chlorine, Ethyne and Water. The compounds’ structures are $O = C = O$ , $Cl - Cl$ , $H - C \equiv C - H$ and $H - O - H$ . So we get that there is only one compound among the options in the options which is having double bonds. So the right option would be Carbon dioxide.
Complete step by step answer:
Option $1$ : $O = C = O$ , The first option is carbon dioxide. There are Double bonds, a total of two double bonds in this structure of compound.
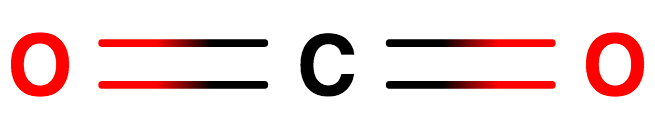
Fig.Structure of Carbon Dioxide having double bond.
Option $2$ : $Cl - Cl$ , The second option is the Salicylic Acid. There is a single bond, but no double bonds in this structure of compound.
Option $3$ : $H - C \equiv C - H$ , The third option is the Ethyne. There are single bonds and triple bonds, but no double bonds in this structure of compound.
Option $4$ : $H - O - H$The fourth option is the Water . There are single bonds, but no double bonds in this structure of compound.
So we get that there is only one compound among the options in the options which is having double bond. So the right option would be Carbon dioxide.
Hence the correct option is option A, Carbon Dioxide.
Note: Carbon dioxide is soluble in water, it occurs naturally in groundwater, rivers and lakes, ice caps, glaciers and seawater. It is present in deposits of petroleum and natural gas. Carbon dioxide has a sharp and acidic odor and generates the taste of soda water in the mouth. However, at normally encountered concentrations it is odorless.
Complete step by step answer:
Option $1$ : $O = C = O$ , The first option is carbon dioxide. There are Double bonds, a total of two double bonds in this structure of compound.
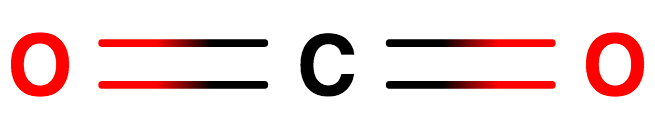
Fig.Structure of Carbon Dioxide having double bond.
Option $2$ : $Cl - Cl$ , The second option is the Salicylic Acid. There is a single bond, but no double bonds in this structure of compound.
Option $3$ : $H - C \equiv C - H$ , The third option is the Ethyne. There are single bonds and triple bonds, but no double bonds in this structure of compound.
Option $4$ : $H - O - H$The fourth option is the Water . There are single bonds, but no double bonds in this structure of compound.
So we get that there is only one compound among the options in the options which is having double bond. So the right option would be Carbon dioxide.
Hence the correct option is option A, Carbon Dioxide.
Note: Carbon dioxide is soluble in water, it occurs naturally in groundwater, rivers and lakes, ice caps, glaciers and seawater. It is present in deposits of petroleum and natural gas. Carbon dioxide has a sharp and acidic odor and generates the taste of soda water in the mouth. However, at normally encountered concentrations it is odorless.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




