
Which of the following correctly depicts the bold line and condensed structure of the compounds?
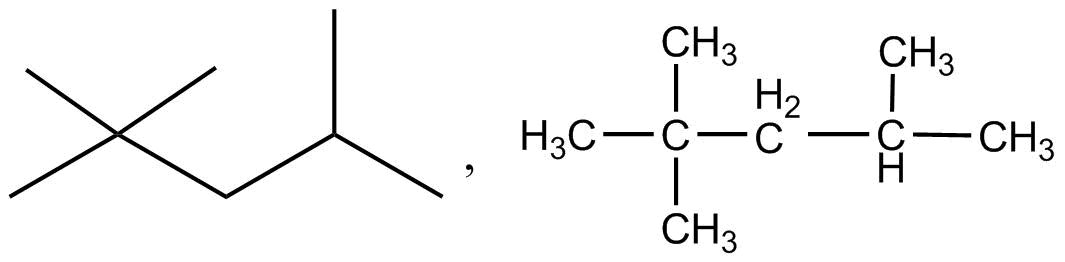
$\left( i \right)2,2,4 - $ Trimethylpentane
$\left( {ii} \right)$ Hexanedial

$\left( {iii} \right)2 - $hydroxypropane$ - 1,2,3 - $tricarboxylic acid

A. $\left( i \right)$
B. $\left( {ii} \right)$
C. $\left( i \right)$ and $\left( {ii} \right)$
D. $\left( i \right),\left( {ii} \right)$ and $\left( {iii} \right)$



Answer
559.2k+ views
Hint: For determining the structure of any organic compound at first the main chain structure needs to be determined. From the main chain structure, the associated structures like the functional groups or the other branching structure in the organic molecule. Calculating the carbon number for the chain of organic molecules the similarity can be checked.
Complete Step by Step Answer:
In the given structure \[\left( i \right)\] the organic molecule has five carbon as part of the main chain of organic molecules. This is observed in the bold line structure of the molecule along with the condensed structure of the organic molecule. There are two methyl $\left( {C{H_3} - } \right)$ groups which are associated with the carbon number $2$ of the main chain of the organic molecule. There is only one methyl $\left( {C{H_3} - } \right)$ group which is associated with the carbon $4$ of the main chain. This structure is the same for both the bold line structure as well as condensed structure proving that in the case of \[\left( i \right)\] the organic molecule structure suits the similar nature.
In the given structure $\left( {ii} \right)$ there are two aldehyde groups on the two sides of both the condensed structure and the bold line structure. In between the $\left( { - CHO} \right)$ groups there are four carbon residues which are depicted in both bold line form as well as the condensed form. This is why the two structures are similar in all aspects with the same main chain and the same functional group associated with the same carbons.
In the given structure $\left( {iii} \right)$ there are two $\left( { - COOH} \right)$ residues which are associated with the three carbon residues forming the main chain. Considering carbon from one side to be the starting carbon, the carbon $3$ has an $\left( { - OH} \right)$ and a $\left( { - COOH} \right)$ group associated with it. This same structure is observed in both but in the bold line diagram the main chain is in horizontal position while in the condensed structure the main chain is in vertical formation.
Therefore, all the given structures are similar according to the two forms given which is why the correct option is, ‘D. $\left( i \right),\left( {ii} \right)$ and $\left( {iii} \right)$’.
Note: Formulating the correct structure of the organic molecule depends on the nature of functional groups involved. Based on the longest chain structure the primary chain of the organic molecule is determined and using the position of the functional group the carbon number can be calculated.
Complete Step by Step Answer:
In the given structure \[\left( i \right)\] the organic molecule has five carbon as part of the main chain of organic molecules. This is observed in the bold line structure of the molecule along with the condensed structure of the organic molecule. There are two methyl $\left( {C{H_3} - } \right)$ groups which are associated with the carbon number $2$ of the main chain of the organic molecule. There is only one methyl $\left( {C{H_3} - } \right)$ group which is associated with the carbon $4$ of the main chain. This structure is the same for both the bold line structure as well as condensed structure proving that in the case of \[\left( i \right)\] the organic molecule structure suits the similar nature.
In the given structure $\left( {ii} \right)$ there are two aldehyde groups on the two sides of both the condensed structure and the bold line structure. In between the $\left( { - CHO} \right)$ groups there are four carbon residues which are depicted in both bold line form as well as the condensed form. This is why the two structures are similar in all aspects with the same main chain and the same functional group associated with the same carbons.
In the given structure $\left( {iii} \right)$ there are two $\left( { - COOH} \right)$ residues which are associated with the three carbon residues forming the main chain. Considering carbon from one side to be the starting carbon, the carbon $3$ has an $\left( { - OH} \right)$ and a $\left( { - COOH} \right)$ group associated with it. This same structure is observed in both but in the bold line diagram the main chain is in horizontal position while in the condensed structure the main chain is in vertical formation.
Therefore, all the given structures are similar according to the two forms given which is why the correct option is, ‘D. $\left( i \right),\left( {ii} \right)$ and $\left( {iii} \right)$’.
Note: Formulating the correct structure of the organic molecule depends on the nature of functional groups involved. Based on the longest chain structure the primary chain of the organic molecule is determined and using the position of the functional group the carbon number can be calculated.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




