
Which of the following currently describes meta-formaldehyde?
A. It is a dimer of \[\text{HCHO}\]
B. It is a trimer of formaldehyde
C. It is a tetramer formaldehyde
D. it is a polymer in which the number of \[\text{HCHO}\] units is more than 100
Answer
589.2k+ views
Hint: The structure of the meta - formaldehyde consists of a ring which has three carbon and three oxygen bonded with each other. Some number of molecules of formaldehyde combine to form a ring structure.
Complete step by step answer:
- In the given question, we have to find the correct statement about the structure of meta - aldehyde.
- As we know that the formula of formaldehyde is \[\text{HCHO}\]and it comes under the group of alcohols.
- When formaldehyde is hydrolysed in the acidic medium, as we know that in hydrolysis reactions the addition of water takes place to break the bonds and form a new product.
- So, the 3 molecules of formaldehyde will combine and form a 6 - membered ring structure in which three atoms of carbon and three atoms oxygen are present.
- The balanced chemical reaction will be:
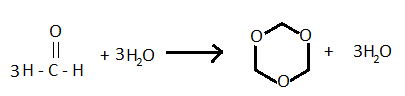
- The compound formed by the hydrolysis of formaldehyde is 1,3,5 trioxane or trioxane.
- As it is made up of three molecules of formaldehyde, it is also known as a trimer of the aldehyde.
-Hence, option B is the correct answer.
- The dimer is the molecule which is made up of only two molecules where tetramer is the compound which is made up of 4 molecules. So, option A and C is an incorrect answer.
- Whereas polymer is made up of more than 100 molecules but trioxane is made up of only 3 molecules. So, Option D is also incorrect.
So, the correct answer is “Option B”.
Note: Trioxane is made from formaldehyde but it can also be used as an anhydrous source for formaldehyde. Anhydrous is the property of the substance to lose the water. The molecular formula of meta- formaldehyde is \[{{\text{C}}_{3}}{{\text{H}}_{6}}{{\text{O}}_{3}}\].
Complete step by step answer:
- In the given question, we have to find the correct statement about the structure of meta - aldehyde.
- As we know that the formula of formaldehyde is \[\text{HCHO}\]and it comes under the group of alcohols.
- When formaldehyde is hydrolysed in the acidic medium, as we know that in hydrolysis reactions the addition of water takes place to break the bonds and form a new product.
- So, the 3 molecules of formaldehyde will combine and form a 6 - membered ring structure in which three atoms of carbon and three atoms oxygen are present.
- The balanced chemical reaction will be:
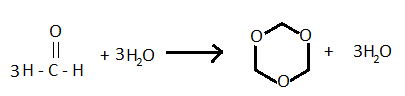
- The compound formed by the hydrolysis of formaldehyde is 1,3,5 trioxane or trioxane.
- As it is made up of three molecules of formaldehyde, it is also known as a trimer of the aldehyde.
-Hence, option B is the correct answer.
- The dimer is the molecule which is made up of only two molecules where tetramer is the compound which is made up of 4 molecules. So, option A and C is an incorrect answer.
- Whereas polymer is made up of more than 100 molecules but trioxane is made up of only 3 molecules. So, Option D is also incorrect.
So, the correct answer is “Option B”.
Note: Trioxane is made from formaldehyde but it can also be used as an anhydrous source for formaldehyde. Anhydrous is the property of the substance to lose the water. The molecular formula of meta- formaldehyde is \[{{\text{C}}_{3}}{{\text{H}}_{6}}{{\text{O}}_{3}}\].
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




