
Which of the following doesn’t give yellow precipitate with $AgN{{O}_{3}}$?
(A) ${{\left( C{{H}_{3}} \right)}_{3}}C-C{{H}_{2}}-I$
(B) $C{{H}_{2}}=CH-C{{H}_{2}}-I$
(C)
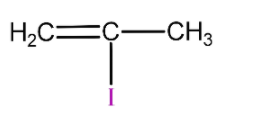
(D)
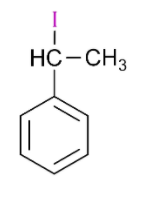


Answer
576.9k+ views
Hint: Think about the reaction which takes place when iodine derivatives react with silver nitrate. Also, take a look at the compounds given in the options and check which one is an exception to this reaction.
Complete step by step solution:
- When iodine derivatives react with silver nitrate, $AgN{{O}_{3}}$, alkyl nitrate is formed which precipitates out as yellow ppt. The reaction is shown as follows:
\[R-I+AgN{{O}_{3}}\to R-ON{{O}_{2}}+AgI\]
- This reaction is readily given by iodine derivatives of alkanes, alkenes, alkynes, allyl and benzyl groups.
- Vinyl iodide and phenyl iodide do not give this reaction due to the presence of strong C-I bond. They do not form any precipitate. Vinyl iodide has conjugate effect and phenyl iodide has resonance effect which stabilizes the bond and makes it stronger.
- Now, let’s take a look at all options given the question.
- ${{\left( C{{H}_{3}} \right)}_{3}}C-C{{H}_{2}}-I$ is an alkane so it will react with silver nitrate to form yellow precipitate.
- $C{{H}_{2}}=CH-C{{H}_{2}}-I$ is allyl iodide, so it will also react with silver nitrate to form yellow precipitate.
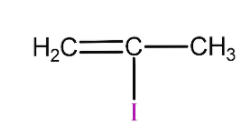
is 2-methyl vinyl iodide so it will not react with silver nitrate due to strong C-I bond and will not form yellow precipitate.
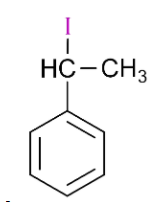
is 1-iodo-1-phenylethane, so it will also react with silver nitrate and form yellow precipitate.
- Therefore, option (C) is the correct answer.
Note: Remember all iodine derivatives except vinyl iodide and phenyl iodide, will react with silver nitrate, $AgN{{O}_{3}}$ to form yellow precipitate. In vinyl iodide, there is conjugate effect and in phenyl iodide, there is resonance effect which increases the C-I bond strength.
Complete step by step solution:
- When iodine derivatives react with silver nitrate, $AgN{{O}_{3}}$, alkyl nitrate is formed which precipitates out as yellow ppt. The reaction is shown as follows:
\[R-I+AgN{{O}_{3}}\to R-ON{{O}_{2}}+AgI\]
- This reaction is readily given by iodine derivatives of alkanes, alkenes, alkynes, allyl and benzyl groups.
- Vinyl iodide and phenyl iodide do not give this reaction due to the presence of strong C-I bond. They do not form any precipitate. Vinyl iodide has conjugate effect and phenyl iodide has resonance effect which stabilizes the bond and makes it stronger.
- Now, let’s take a look at all options given the question.
- ${{\left( C{{H}_{3}} \right)}_{3}}C-C{{H}_{2}}-I$ is an alkane so it will react with silver nitrate to form yellow precipitate.
- $C{{H}_{2}}=CH-C{{H}_{2}}-I$ is allyl iodide, so it will also react with silver nitrate to form yellow precipitate.

is 2-methyl vinyl iodide so it will not react with silver nitrate due to strong C-I bond and will not form yellow precipitate.

is 1-iodo-1-phenylethane, so it will also react with silver nitrate and form yellow precipitate.
- Therefore, option (C) is the correct answer.
Note: Remember all iodine derivatives except vinyl iodide and phenyl iodide, will react with silver nitrate, $AgN{{O}_{3}}$ to form yellow precipitate. In vinyl iodide, there is conjugate effect and in phenyl iodide, there is resonance effect which increases the C-I bond strength.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




