
Which of the following form a stable hemiketal?
(A)

(B)

(C)
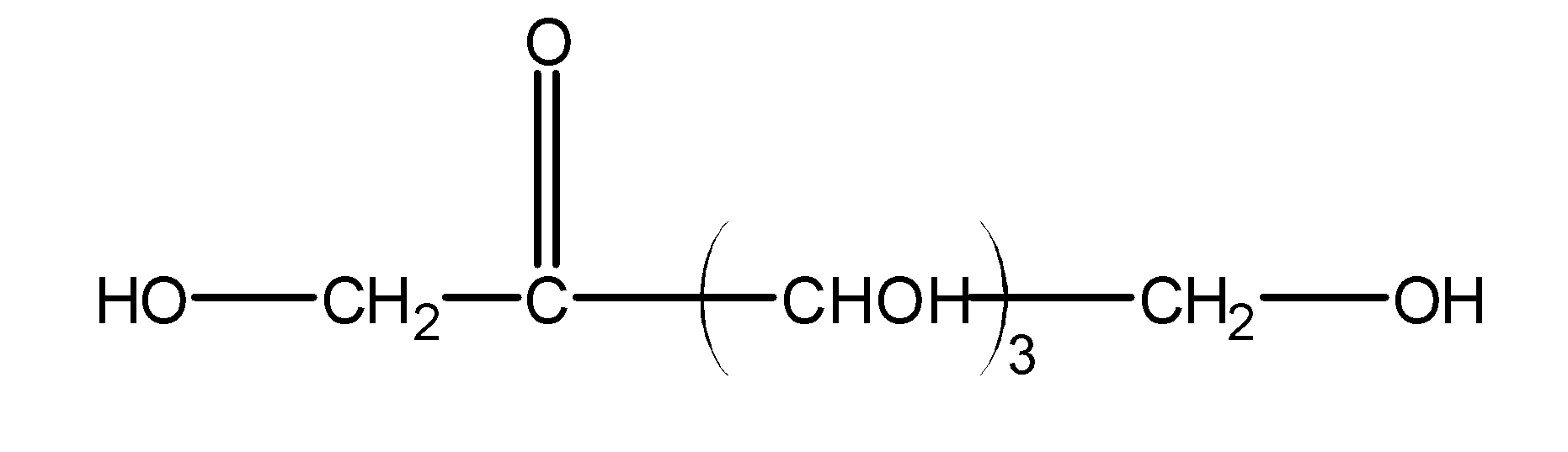
(D)

| (A) | 
|
| (B) | 
|
| (C) | 
|
| (D) | 
|
Answer
542.4k+ views
Hint: Hemiketals are the organic compounds that are formed by the addition of an alcohol to a carbonyl group or specifically to ketone. It is formed by the attack of an internal alcohol group to the carbonyl group present in the same chain.
Complete answer:
Hemiketals in organic chemistry are the compounds that are formed by the attack of the internal alcohol group on the ketone group.
Similarly, hemiacetals are the organic compounds that are formed by the attack of the internal alcohol group on the aldehyde group.
The more carbon atoms present in the chain, the more stable the hemiketal is.
Similarly, for the hemiacetals, if more numbers of carbon atoms are present in the chain, hemiacetal will be more stable.
However, if the carbonyl compound is stabilised by resonance of the ring structures present in the chain, then hemiketal will be unstable.
If we look at compound A

The carbonyl chain will be stabilised by the presence of two rings of phenol, hence the hemiketal will be unstable in this compound.
Looking at the structure B
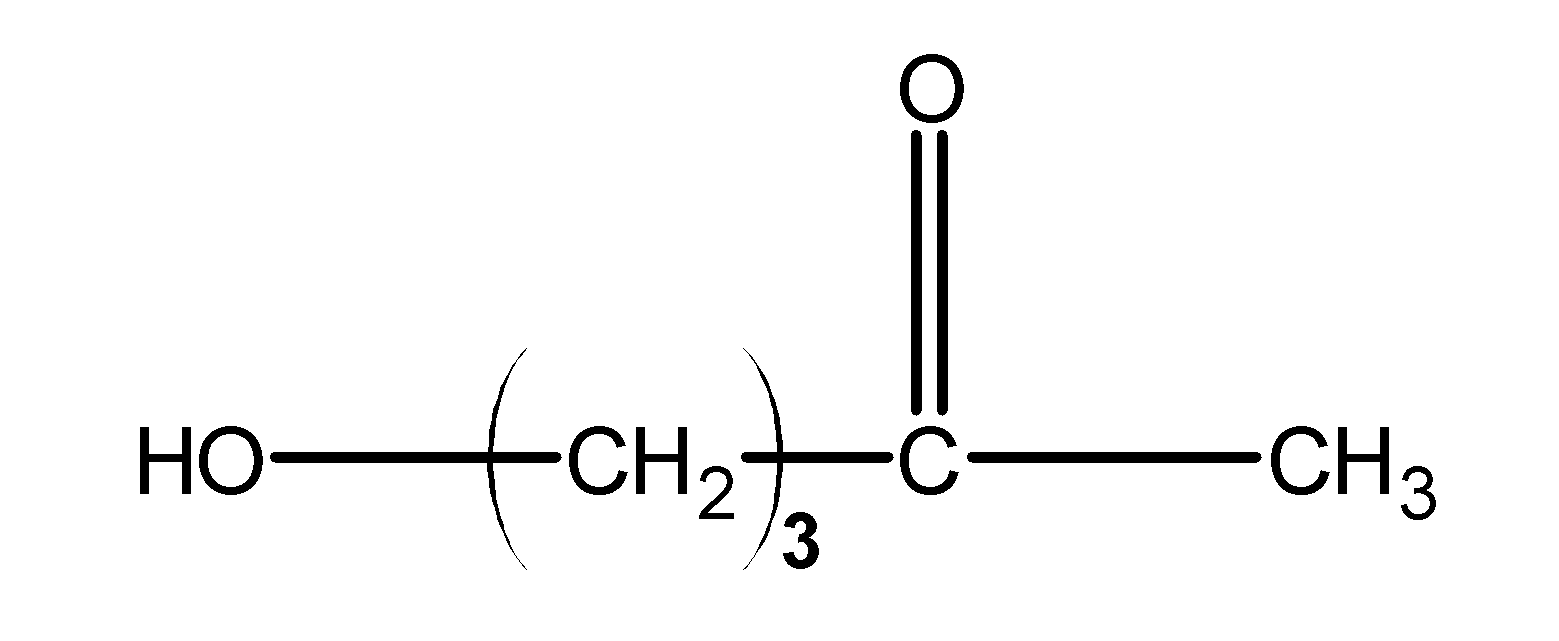
The O from OH shares its lone pair with the carbon atom of the ketone and forms a hemiacetal. This structure forms a 5 carbon ring. Thus, it becomes a stable hemiacetal.
In structure C

It is a 6 membered ring, often stable hemiketals are formed by 4 membered carbon chain or 5 membered carbon chain. Thus, it forms an unstable hemiketal.
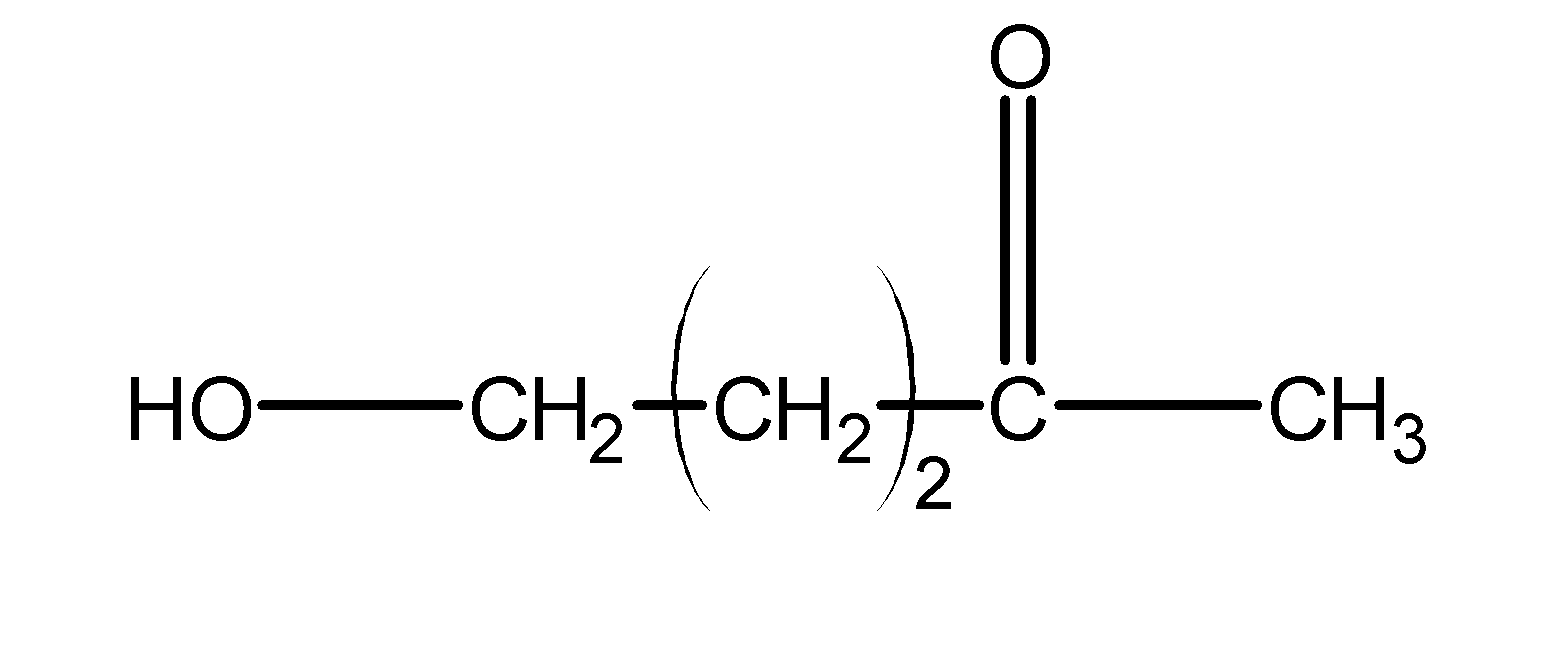
The above chain is a 5-carbon membered ring and contains a carbon as well as a ketone. Thus, it forms a stable hemiacetal ring.
Hence, (D) is the correct option.
Note:
Hemiketals are the compounds that are bonded with an alkoxy group on one side, alcohol on another side and the other two bonds contain carbon chain rings. A stable hemiketal is generally formed by 4 or 5 membered rings.
Complete answer:
Hemiketals in organic chemistry are the compounds that are formed by the attack of the internal alcohol group on the ketone group.
Similarly, hemiacetals are the organic compounds that are formed by the attack of the internal alcohol group on the aldehyde group.
The more carbon atoms present in the chain, the more stable the hemiketal is.
Similarly, for the hemiacetals, if more numbers of carbon atoms are present in the chain, hemiacetal will be more stable.
However, if the carbonyl compound is stabilised by resonance of the ring structures present in the chain, then hemiketal will be unstable.
If we look at compound A

The carbonyl chain will be stabilised by the presence of two rings of phenol, hence the hemiketal will be unstable in this compound.
Looking at the structure B

The O from OH shares its lone pair with the carbon atom of the ketone and forms a hemiacetal. This structure forms a 5 carbon ring. Thus, it becomes a stable hemiacetal.
In structure C

It is a 6 membered ring, often stable hemiketals are formed by 4 membered carbon chain or 5 membered carbon chain. Thus, it forms an unstable hemiketal.

The above chain is a 5-carbon membered ring and contains a carbon as well as a ketone. Thus, it forms a stable hemiacetal ring.
Hence, (D) is the correct option.
Note:
Hemiketals are the compounds that are bonded with an alkoxy group on one side, alcohol on another side and the other two bonds contain carbon chain rings. A stable hemiketal is generally formed by 4 or 5 membered rings.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




