
Which of the following gases requires the least temperature for liquefaction?
A.Ammonia
B.Helium
C.Hydrogen
D.Nitrogen
Answer
579.6k+ views
Hint: Basically, liquefaction of gases is the physical conversion of gas into liquid state. Moreover, when pressure on a gas increases, then the molecules come closer and hence the temperature gets reduced which removes enough energy to make it change from gaseous to liquid state.
Complete step by step answer:
Thomas Andres was the scientist who investigated the complete relationship between volume-temperature and pressure of a substance in gaseous as well as in liquid state by studying the behavior of carbon dioxide. Further, when the temperature of the gas is decreased then the curve shows deviation from the ideal one. The curve is as shown:
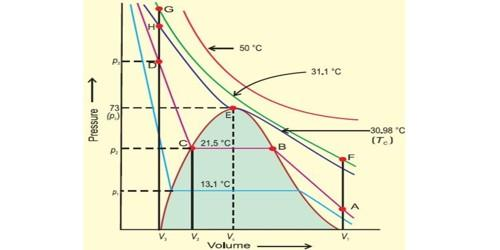
Now, ammonia gas can be liquefied by applying high pressure and lowering the temperature. Further, any gas can be liquefied when the gas has been exposed to high pressures and low temperatures. The pressure that has to be applied as well as the temperature depends on the molecular forces of the gas molecules. Therefore, higher the intermolecular forces, higher is the temperature for liquefaction and helium has the least intermolecular forces while ammonia has the highest intermolecular forces. So, helium has the least temperature for liquefaction.
Hence, option B is correct.
Note: There is a limit to the temperature where the pressure has to be applied for the liquefaction of the gas. This temperature is known as critical temperature. It is basically the highest temperature at which the substance can exist as a liquid. Moreover, at temperatures above the critical temperature, the substance can no longer be liquefied, regardless of the amount of pressure applied to it.
Complete step by step answer:
Thomas Andres was the scientist who investigated the complete relationship between volume-temperature and pressure of a substance in gaseous as well as in liquid state by studying the behavior of carbon dioxide. Further, when the temperature of the gas is decreased then the curve shows deviation from the ideal one. The curve is as shown:

Now, ammonia gas can be liquefied by applying high pressure and lowering the temperature. Further, any gas can be liquefied when the gas has been exposed to high pressures and low temperatures. The pressure that has to be applied as well as the temperature depends on the molecular forces of the gas molecules. Therefore, higher the intermolecular forces, higher is the temperature for liquefaction and helium has the least intermolecular forces while ammonia has the highest intermolecular forces. So, helium has the least temperature for liquefaction.
Hence, option B is correct.
Note: There is a limit to the temperature where the pressure has to be applied for the liquefaction of the gas. This temperature is known as critical temperature. It is basically the highest temperature at which the substance can exist as a liquid. Moreover, at temperatures above the critical temperature, the substance can no longer be liquefied, regardless of the amount of pressure applied to it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




