
Which of the following has cross-linked polymer chains?
A.Bakelite
B.Polyester
C.PVC
D.Nylon
Answer
506.7k+ views
Hint: For answering this question we should know about cross-link polymers. Cross-linked means a type of bond which connects one polymer chain to the other polymer chain. Cross-linked polymers are those polymers which form a cross-link bond between their monomeric units.
Complete answer:
Let’s see each option one by one and see how they are formed to identify them
First is Bakelite it is formed by Phenol and Formaldehyde, the reaction involved is given as
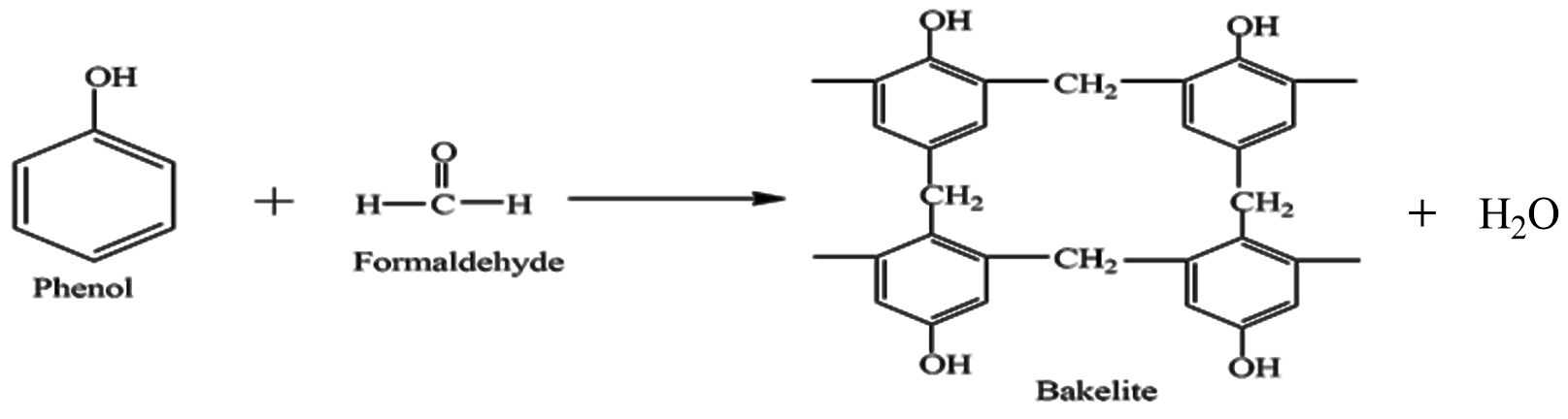
We can see Phenol and Formaldehyde are reacted, they form a cross-link and Bakelite is formed. It is a thermosetting plastic possessing permanent shape and size, these polymers are highly cross-linked causing it to be strong and hard. It is a poor conductor of heat and electricity and is used in switches, sockets and frying pan handles.
Second option is polyester. It is formed by \[benzene - 1,4 - dicarboxylic acid\] and \[ethane - 1,2 - diol\] it is a condensation polymer. See the reaction below
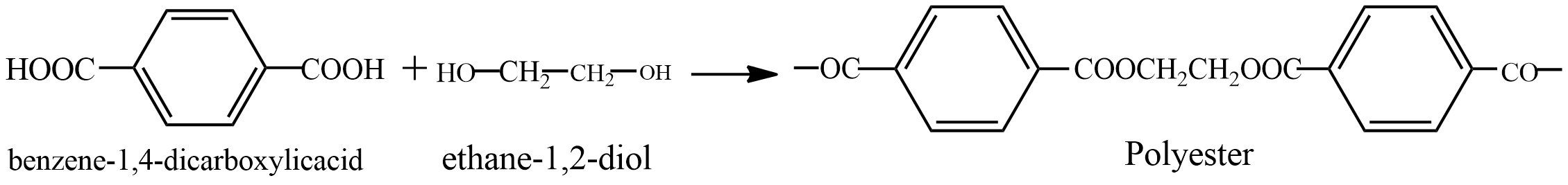
In this reaction molecules of water are also formed. It is a thermoplastic, used widely in clothing.
Next option is PVC it prepared by polymerisation Vinyl chloride let’s see the reaction
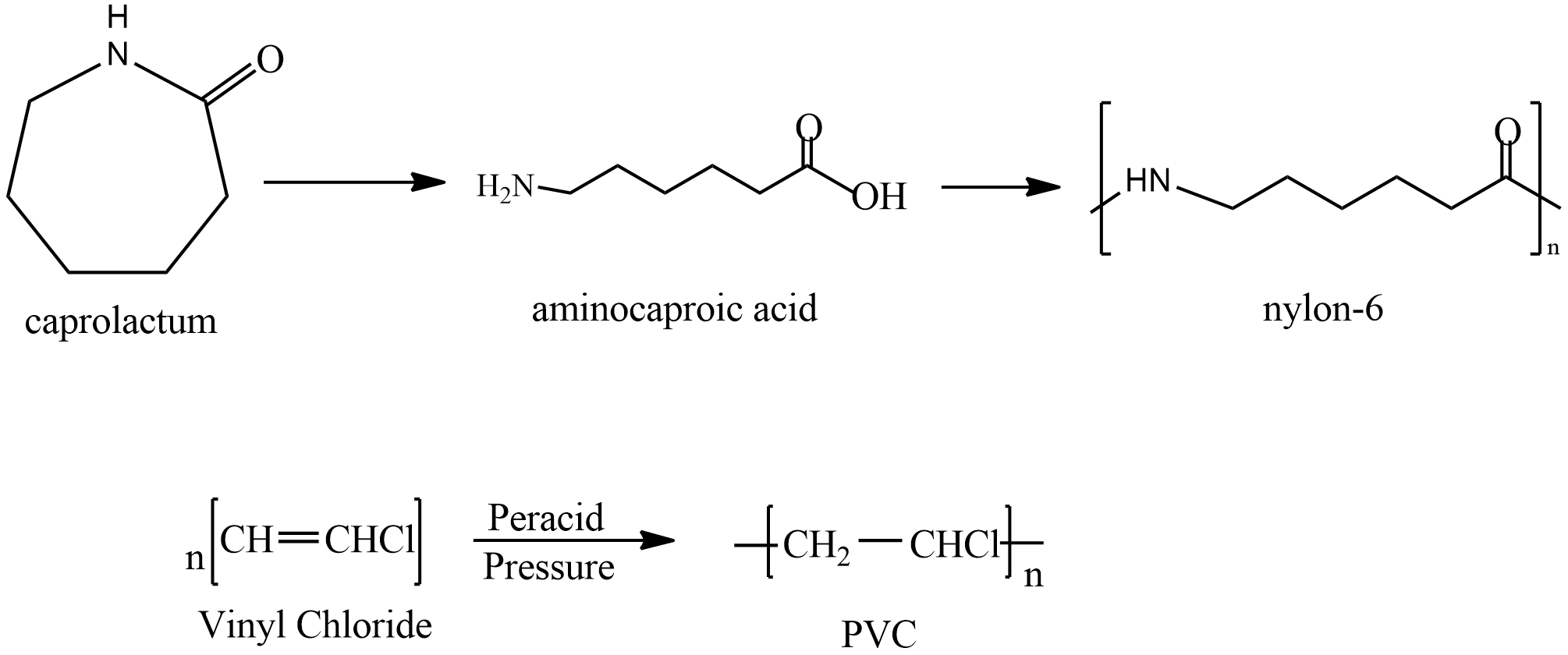
It is used in making sewage pipes and places where corrosion is to be avoided. It is a good insulator and is used to insulate wires.
Last is nylon; it is prepared by polymerization of Caprolactam. The reaction is given as
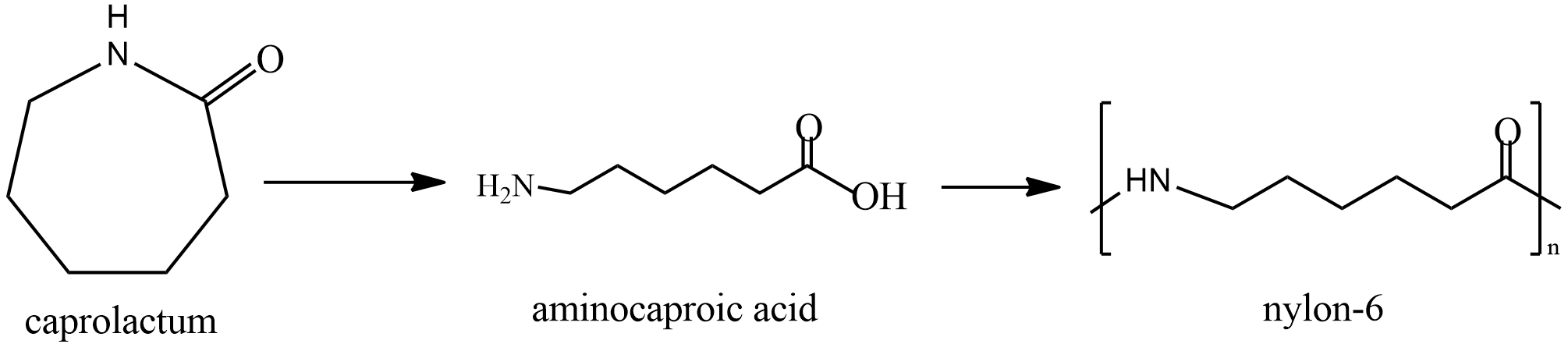
It is first converted to Aminocaproic acid and then Nylon is formed. It is used in clothing and other industries in making seat belts, nets, parachutes etc.
From all the reactions we can see that only Bakelite forms Cross-links so option A is the right answer.
Note:
The cross-linked polymers are long chain polymers which are either linear or branched. The covalent bonds formed in these cross-link polymers are strong due to intermolecular forces attracting other polymer chains resulting in stronger and more stable compounds.
Complete answer:
Let’s see each option one by one and see how they are formed to identify them
First is Bakelite it is formed by Phenol and Formaldehyde, the reaction involved is given as

We can see Phenol and Formaldehyde are reacted, they form a cross-link and Bakelite is formed. It is a thermosetting plastic possessing permanent shape and size, these polymers are highly cross-linked causing it to be strong and hard. It is a poor conductor of heat and electricity and is used in switches, sockets and frying pan handles.
Second option is polyester. It is formed by \[benzene - 1,4 - dicarboxylic acid\] and \[ethane - 1,2 - diol\] it is a condensation polymer. See the reaction below

In this reaction molecules of water are also formed. It is a thermoplastic, used widely in clothing.
Next option is PVC it prepared by polymerisation Vinyl chloride let’s see the reaction

It is used in making sewage pipes and places where corrosion is to be avoided. It is a good insulator and is used to insulate wires.
Last is nylon; it is prepared by polymerization of Caprolactam. The reaction is given as

It is first converted to Aminocaproic acid and then Nylon is formed. It is used in clothing and other industries in making seat belts, nets, parachutes etc.
From all the reactions we can see that only Bakelite forms Cross-links so option A is the right answer.
Note:
The cross-linked polymers are long chain polymers which are either linear or branched. The covalent bonds formed in these cross-link polymers are strong due to intermolecular forces attracting other polymer chains resulting in stronger and more stable compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




