
Which of the following have planar structure?
This question has multiple correct options
A.${I_3}^ - $
B.${H_2}O$
C.$PC{l_5}$
D.$Xe{F_4}$
Answer
570k+ views
Hint: Each molecule exhibits different geometry with respect to their bonding. Hybridization is the method used to determine the geometry. To check which molecule exhibits which geometry we will check for each.
Complete answer:
Let’s consider the first molecule ${I_3}^ - $, this molecule exhibits linear geometry. There are three iodine atoms paired together, one atom has a negative charge due to which there are 3 lone pairs. Thus, the orientation will be linear with each atom at $180^\circ$ angle with one another.
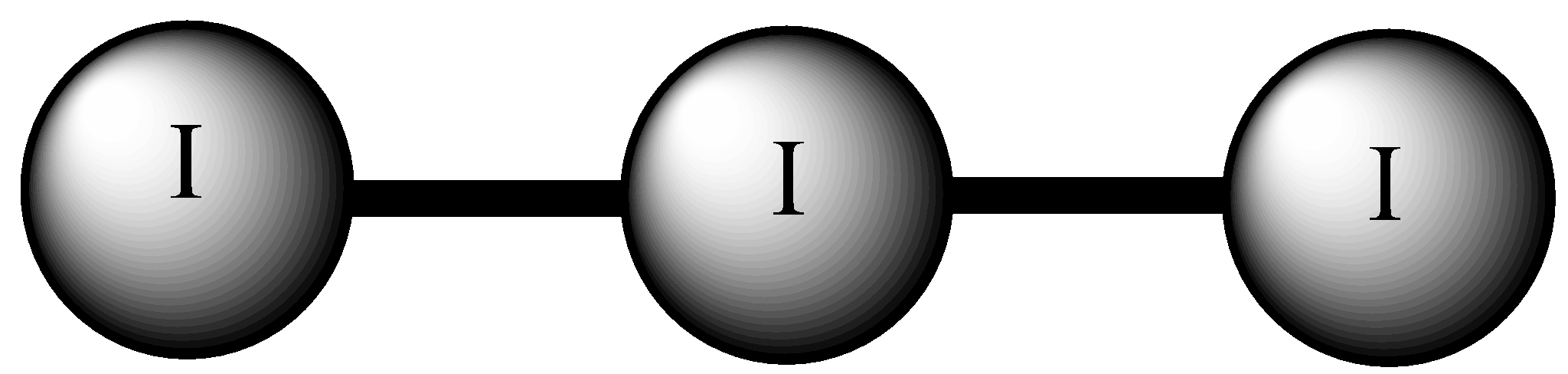
Thus, this is one correct answer.
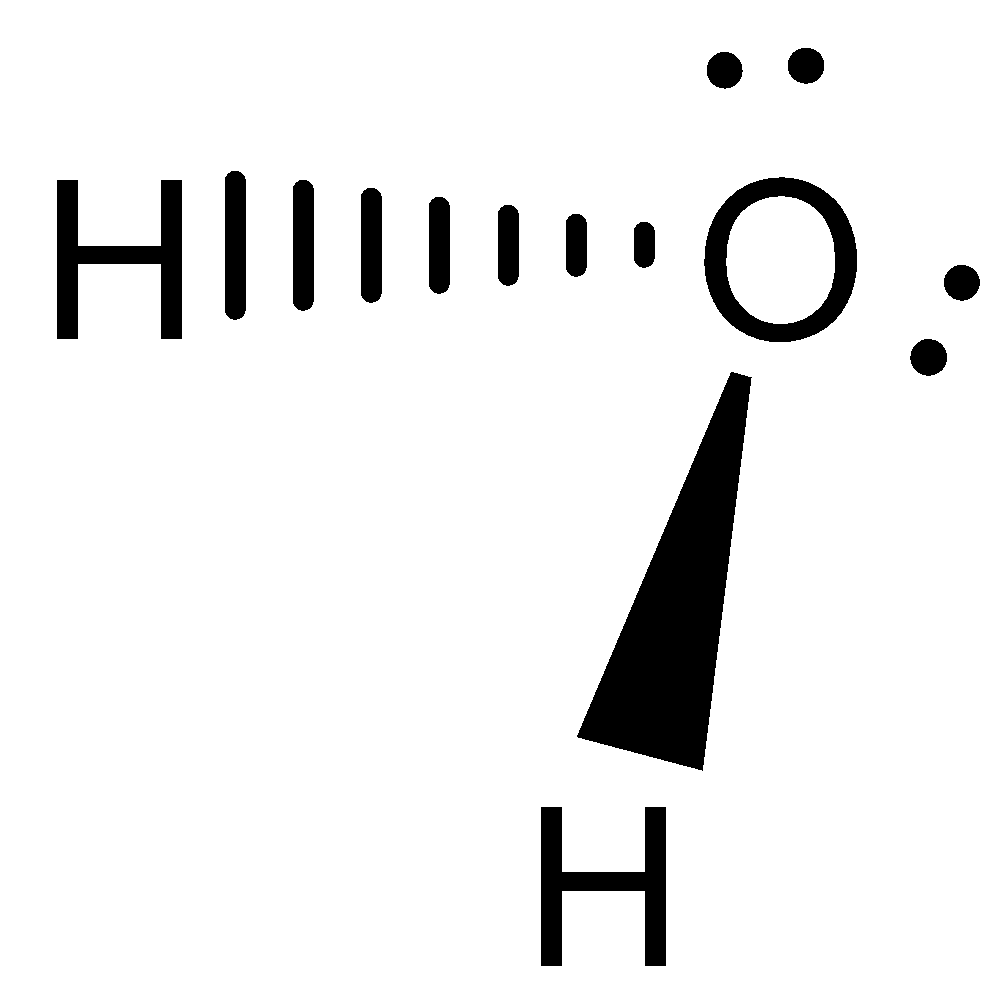
Let us consider the next molecule is ${H_2}O$. Water molecules have 4 electron density, with 2 bonded pairs and 2 lone pairs. Hence, it exhibits in bent shape and not planar. The geometry can be visualised as below,
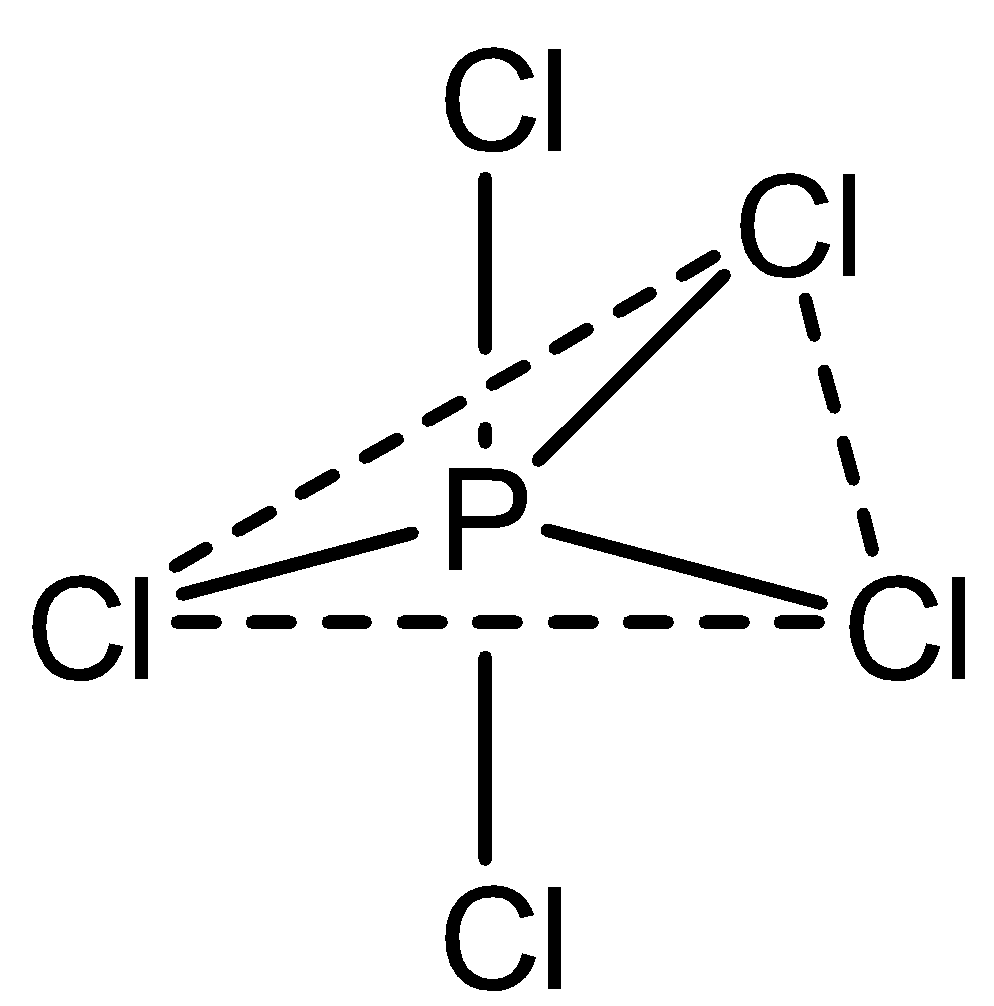
In the case of $PC{l_5}$, phosphorus atoms are bonded with 5 chlorine atoms. It exhibits a trigonal bipyramidal structure which is not planar, with three atoms in the planar structure and one above and one below at the tip of the hedral structure.
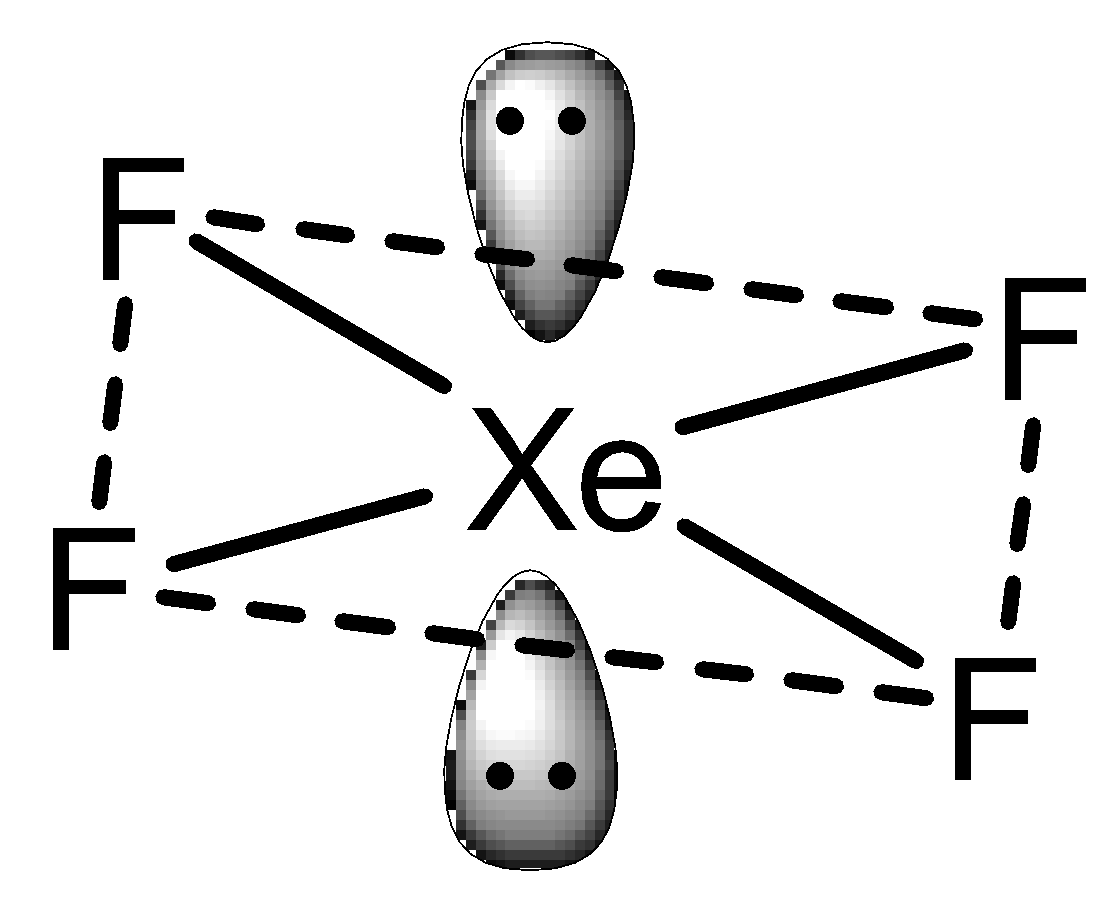
In the case of $Xe{F_4}$ which exhibits $s{p^3}{d^2}$ hybridization but in a square planar geometry with four fluorine atoms at the corner of the square plane and two lone pairs. Thus, the structure can be shown as,
Thus, the correct answer to the question is option A and D.
Note: We have to remember that the hybridization depends on the bonding of an atom with respect to the molecules present. Some atoms carry lone pairs even after bonding like oxygen. Therefore, their structures get modified due to the presence of lone pairs. As they tend to repel from each other due to a strong negative charge. In questions like this, it is important to determine the structure of each molecule.
Complete answer:
Let’s consider the first molecule ${I_3}^ - $, this molecule exhibits linear geometry. There are three iodine atoms paired together, one atom has a negative charge due to which there are 3 lone pairs. Thus, the orientation will be linear with each atom at $180^\circ$ angle with one another.
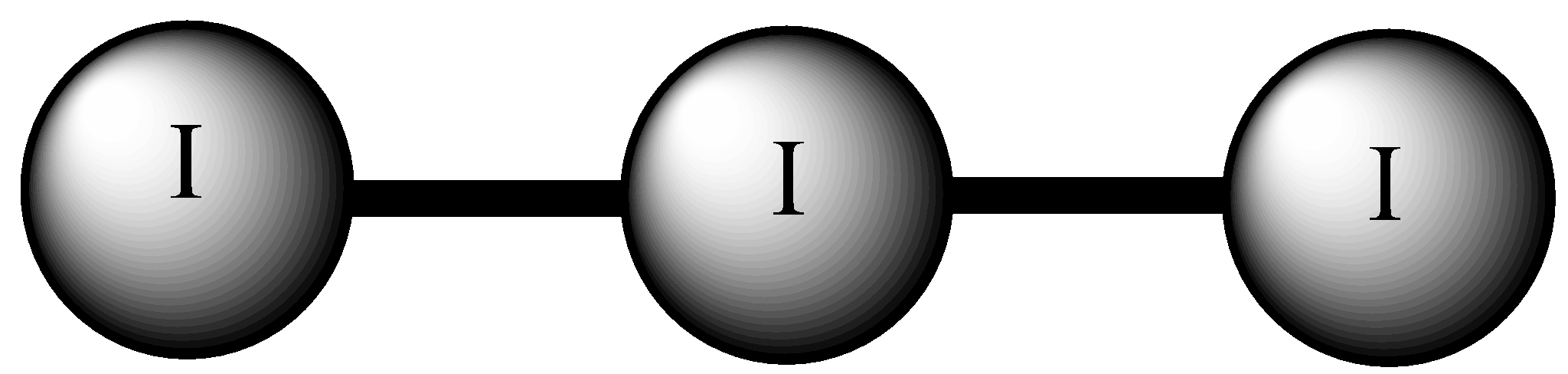
Thus, this is one correct answer.
Let us consider the next molecule is ${H_2}O$. Water molecules have 4 electron density, with 2 bonded pairs and 2 lone pairs. Hence, it exhibits in bent shape and not planar. The geometry can be visualised as below,

In the case of $PC{l_5}$, phosphorus atoms are bonded with 5 chlorine atoms. It exhibits a trigonal bipyramidal structure which is not planar, with three atoms in the planar structure and one above and one below at the tip of the hedral structure.

In the case of $Xe{F_4}$ which exhibits $s{p^3}{d^2}$ hybridization but in a square planar geometry with four fluorine atoms at the corner of the square plane and two lone pairs. Thus, the structure can be shown as,

Thus, the correct answer to the question is option A and D.
Note: We have to remember that the hybridization depends on the bonding of an atom with respect to the molecules present. Some atoms carry lone pairs even after bonding like oxygen. Therefore, their structures get modified due to the presence of lone pairs. As they tend to repel from each other due to a strong negative charge. In questions like this, it is important to determine the structure of each molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




