
Which of the following hydride is electron-precise hydride?
A.${{B}_{2}}{{H}_{6}}$
B.$N{{H}_{3}}$
C.${{H}_{2}}O$
D.$C{{H}_{4}}$
Answer
565.5k+ views
Hint:To solve this question, first we should have prior knowledge about the octet configuration. According to the octet configuration as the name suggests Oct means eight the atom has eight electrons in the outermost orbit.
Complete answer:
In the year 1904, Richard Abegg proposed the concept of coordination number and said that the atoms behave as donors or acceptors of electrons. The octet rule uses this idea and states that every atom binds with another atom to have eight electrons in its outermost shell.
The octet rule is able to explain the formation of a number of compounds and also able to explain the stability of the compound.
Electron deficient compounds have a lesser number of electrons which are generally required for writing the Lewis structures. The hydrides of group 13 generally form electron deficient species. The most common example of electron deficient species is ${{B}_{2}}{{H}_{6}}$.
In ${{B}_{2}}{{H}_{6}}$ ,Valence electrons in boron = 3
Boron requires 5 electrons to complete its octet. Each boron atom uses 2 of its electrons to form bonds with the terminal hydrogens but still the octet of boron is incomplete.
The structure of ${{B}_{2}}{{H}_{6}}$ is mentioned below:
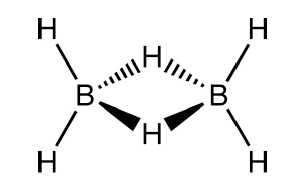
Hence, the correct answer is option (A).
Note:
The octet rule is based on the concept that electronic configuration of the noble gases are most stable and they have eight electrons in their outermost orbital so every element should attain the similar electronic configuration to become stable.
Complete answer:
In the year 1904, Richard Abegg proposed the concept of coordination number and said that the atoms behave as donors or acceptors of electrons. The octet rule uses this idea and states that every atom binds with another atom to have eight electrons in its outermost shell.
The octet rule is able to explain the formation of a number of compounds and also able to explain the stability of the compound.
Electron deficient compounds have a lesser number of electrons which are generally required for writing the Lewis structures. The hydrides of group 13 generally form electron deficient species. The most common example of electron deficient species is ${{B}_{2}}{{H}_{6}}$.
In ${{B}_{2}}{{H}_{6}}$ ,Valence electrons in boron = 3
Boron requires 5 electrons to complete its octet. Each boron atom uses 2 of its electrons to form bonds with the terminal hydrogens but still the octet of boron is incomplete.
The structure of ${{B}_{2}}{{H}_{6}}$ is mentioned below:

Hence, the correct answer is option (A).
Note:
The octet rule is based on the concept that electronic configuration of the noble gases are most stable and they have eight electrons in their outermost orbital so every element should attain the similar electronic configuration to become stable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




