
Which of the following illustrations represents a pure substance? Why?
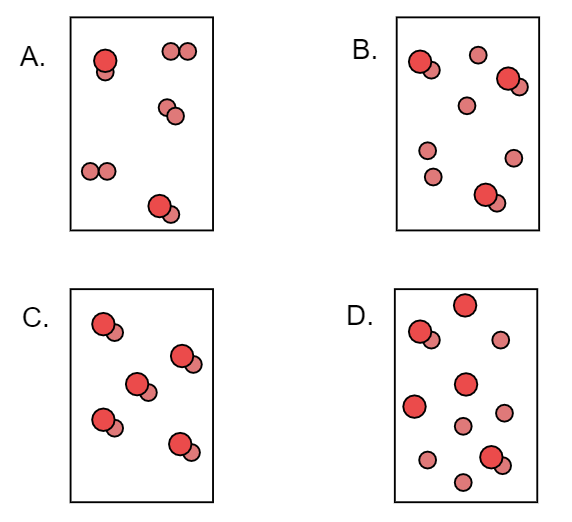

Answer
487.2k+ views
Hint: Pure substances are those substances which are made up of only one type of element. It may be the same type of diatomic molecule or it may be the same kind of heteroatomic molecule. Basically it will contain the same type of molecules of compounds. Thus we will analyze each given option and identify the pure substance of the same molecule.
Complete Step By Step Answer:
Pure substances are those substances which have the same kind of molecules or atoms in them. They may have diatomic molecules or heteroatomic molecules. But the whole substance should be made of that same molecule. We will observe each option respectively. Let us assume that:
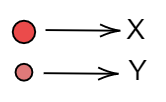
Now we can easily find the pure substance.
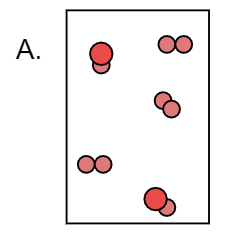
Here we can observe that the Y atom forms a diatomic molecule with itself and also it forms XY. Thus we can say that it is not a pure substance.
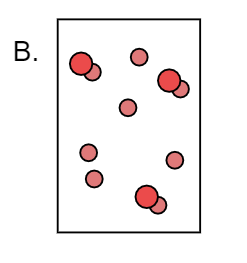
Here we can observe that X forms a compound with Y and forms XY. Also X atoms are present separately. Thus it is a combination of both XY and X. Therefore it is not a pure substance.
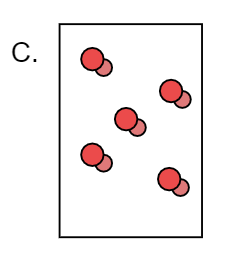
Here we can observe that X forms a compound with Y and forms XY. Here only the XY molecule is present. There it is pure substance because none other molecule except XY is present here.
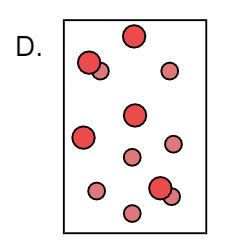
Here we can observe that X forms a compound with Y and forms XY. Also there are X and Y atoms too present. Thus it is a mixture of XY, X and Y. Therefore it is not a pure substance.
Hence we can say that pure substance is C.
Note:
Pure substances generally have a lower melting point than impure substances. This is because the force of attraction is greater in impure substance. Impurity also arises due to the presence of different atoms in the given substance. Thus a substance which contains the same kind of molecule is known as a pure substance.
Complete Step By Step Answer:
Pure substances are those substances which have the same kind of molecules or atoms in them. They may have diatomic molecules or heteroatomic molecules. But the whole substance should be made of that same molecule. We will observe each option respectively. Let us assume that:

Now we can easily find the pure substance.

Here we can observe that the Y atom forms a diatomic molecule with itself and also it forms XY. Thus we can say that it is not a pure substance.

Here we can observe that X forms a compound with Y and forms XY. Also X atoms are present separately. Thus it is a combination of both XY and X. Therefore it is not a pure substance.

Here we can observe that X forms a compound with Y and forms XY. Here only the XY molecule is present. There it is pure substance because none other molecule except XY is present here.

Here we can observe that X forms a compound with Y and forms XY. Also there are X and Y atoms too present. Thus it is a mixture of XY, X and Y. Therefore it is not a pure substance.
Hence we can say that pure substance is C.
Note:
Pure substances generally have a lower melting point than impure substances. This is because the force of attraction is greater in impure substance. Impurity also arises due to the presence of different atoms in the given substance. Thus a substance which contains the same kind of molecule is known as a pure substance.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




