
Which of the following is a homopolymer?
A.Bakelite
B. Nylon 6,6
C. Neoprene
D.Buna-S
Answer
585.6k+ views
Hint:All the compounds mentioned above are monomers. Under the right conditions, they undergo polymerization and form long-chained polymers. The properties of these polymers vary depending on the nature of the monomer, the type of branching, etc. Each polymer has a specific set of use cases following its properties.
Complete step by step answer:
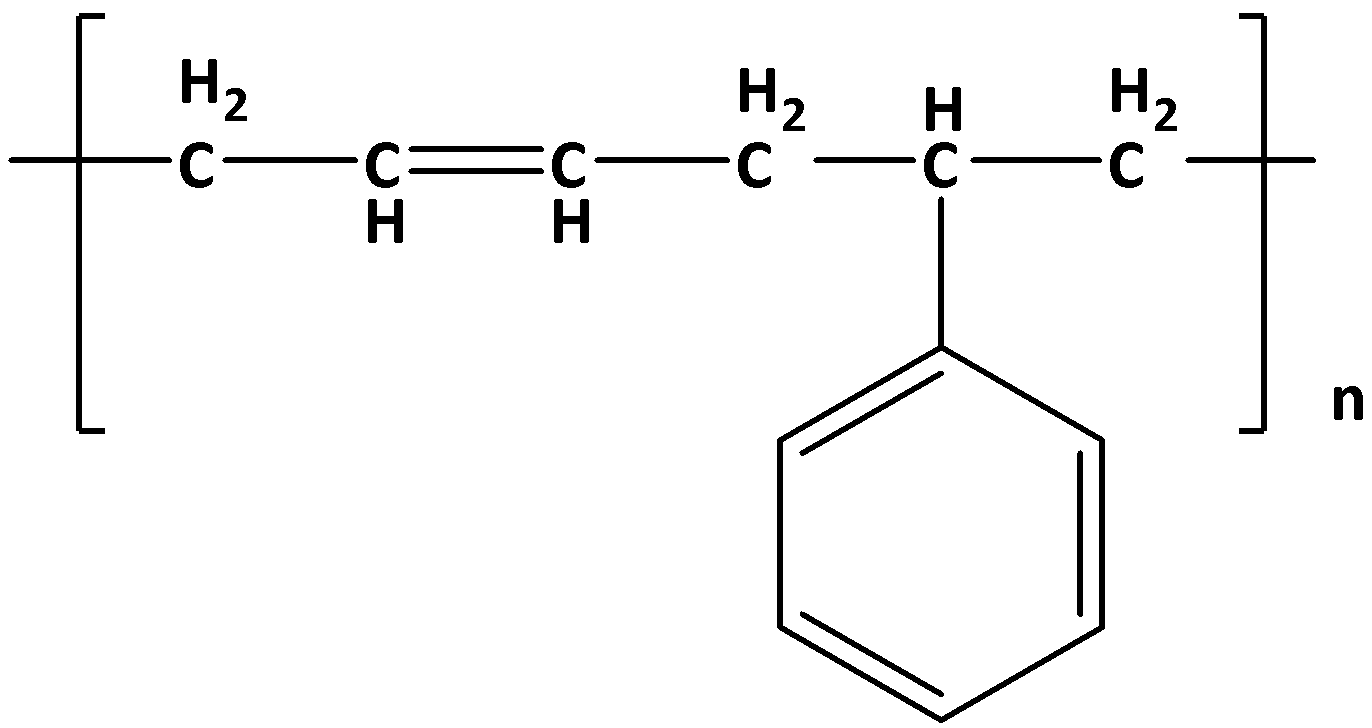
Buna – S is a random copolymer that is formed by the emulsion polymerization of a mixture of butadiene and styrene in the ratio of \[1:3\] . This reaction takes place in the presence of a peroxide catalyst at \[5^\circ C\] and therefore the product is called cold rubber.
The structure of Buna – S is given by:
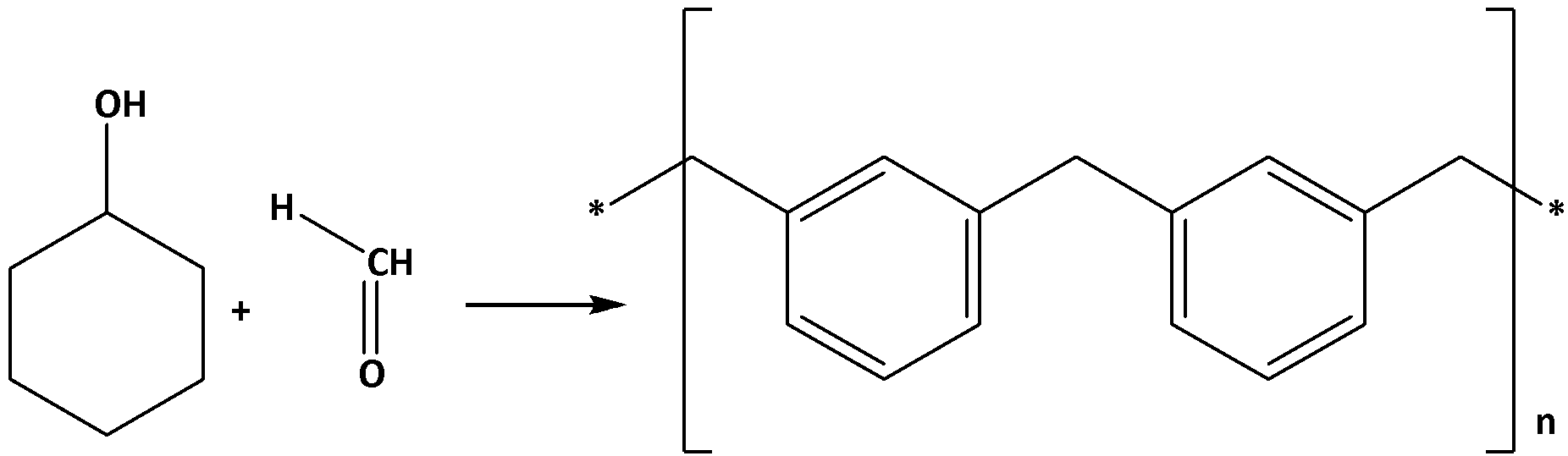
Bakelite is a copolymer, which is produced in a condensation reaction. phenol and formaldehyde are reacted with a certain filler material under the conditions of high pressure and temperature. The chemical reaction for the production of Bakelite can be given as follows:
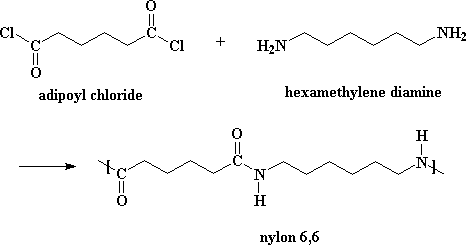
To prepare copolymer Nylon 6,6 adipic acid and hexamethylenediamine are heated at 553 K under pressure.
This reaction is shown:
Neoprene can be prepared from chloroprene. The reaction is shown below,
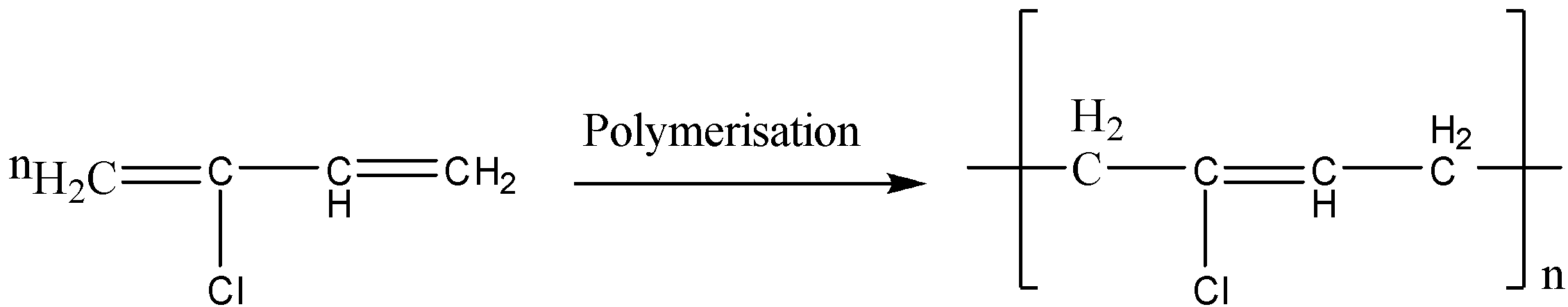
Neoprene is the homopolymer.
Hence option C is correct.
Note:
Neoprene is similar to isoprene, neoprene too has mechanical properties similar to rubber. Contrary to many other materials that chafe and deteriorate over long term usage, neoprene manages to maintain its shape. Hence, it cannot be used in non-stick cooking vessels.
Complete step by step answer:
Buna – S is a random copolymer that is formed by the emulsion polymerization of a mixture of butadiene and styrene in the ratio of \[1:3\] . This reaction takes place in the presence of a peroxide catalyst at \[5^\circ C\] and therefore the product is called cold rubber.
The structure of Buna – S is given by:

Bakelite is a copolymer, which is produced in a condensation reaction. phenol and formaldehyde are reacted with a certain filler material under the conditions of high pressure and temperature. The chemical reaction for the production of Bakelite can be given as follows:

To prepare copolymer Nylon 6,6 adipic acid and hexamethylenediamine are heated at 553 K under pressure.
This reaction is shown:

Neoprene can be prepared from chloroprene. The reaction is shown below,

Neoprene is the homopolymer.
Hence option C is correct.
Note:
Neoprene is similar to isoprene, neoprene too has mechanical properties similar to rubber. Contrary to many other materials that chafe and deteriorate over long term usage, neoprene manages to maintain its shape. Hence, it cannot be used in non-stick cooking vessels.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




