
Which of the following is a tertiary alcohol
(A) Neopentyl alcohol
(B) Isopropyl alcohol
(C) Carbinol
(D) 2-methylbutan-2-ol
Answer
589.8k+ views
Hint: Drawing the structures of given compounds, we will get to know the hydroxyl group is attached to how many other alkyl groups. Since we need a tertiary alcohol, there would be three alkyl groups attached to the carbon atom holding hydroxyl group. Hence by looking at the structures, we could differentiate between primary, secondary and tertiary alcohols.
Complete answer:
- As we know alcohols are those organic compounds which contain one, two or more hydroxyl groups(-OH) that are attached to the carbon atom in a hydrocarbon chain.
- There are three types of alcohol and they are classified as primary, secondary and tertiary alcohols. This classification is done in accordance to where the carbon atom of an alkyl group is attached to the hydroxyl group.
- The primary alcohols are those alcohols in which the carbon atom of the hydroxyl group is attached to only one single alkyl group.
- Similarly, secondary alcohols are those alcohols in which the carbon atom of the hydroxyl group is attached to two alkyl groups on either side and in tertiary alcohols, the carbon atom of hydroxyl group is attached to three alkyl groups.
-Therefore, by looking at the structures of the given compounds one can identify whether the alcohol is primary, secondary or tertiary.
- Let's start with neopentyl alcohol. Its structure is given below
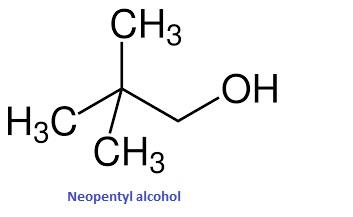
As we can see here, the carbon atom holding the OH group is attached directly to only one carbon atom. Hence, it’s a primary alcohol.
- Isopropyl alcohol has the following structure
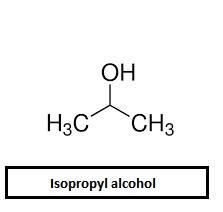
In isopropyl alcohol, the carbon that is attached with OH group is attached to two carbon atoms and thus it’s a secondary alcohol.
- Carbinol is another name for methanol ($C{{H}_{3}}OH$)
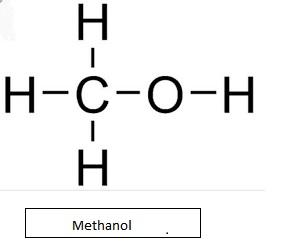
Even though there are no alkyl groups directly linked to the carbon atom, it's also considered as primary alcohol.
- The only remaining option is 2-methylbutan-2-ol. Its structure is given as
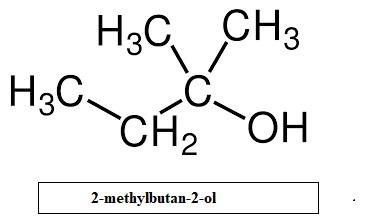
Here, the carbon atom holding the OH group is attached directly to three alkyl groups. Thus 2-methylbutan-2-ol is a tertiary alcohol.
Therefore, the answer is option (D) 2-methylbutan-2-ol .
Note:
The type of alcohol can also be determined by counting the number of hydrogen atoms bonded to the carbon with the OH group. Primary alcohols have a general formula of R$C{{H}_{2}}OH$, secondary alcohols have ${{R}_{2}}CHOH$ and tertiary alcohols have ${{R}_{3}}COH$.
Complete answer:
- As we know alcohols are those organic compounds which contain one, two or more hydroxyl groups(-OH) that are attached to the carbon atom in a hydrocarbon chain.
- There are three types of alcohol and they are classified as primary, secondary and tertiary alcohols. This classification is done in accordance to where the carbon atom of an alkyl group is attached to the hydroxyl group.
- The primary alcohols are those alcohols in which the carbon atom of the hydroxyl group is attached to only one single alkyl group.
- Similarly, secondary alcohols are those alcohols in which the carbon atom of the hydroxyl group is attached to two alkyl groups on either side and in tertiary alcohols, the carbon atom of hydroxyl group is attached to three alkyl groups.
-Therefore, by looking at the structures of the given compounds one can identify whether the alcohol is primary, secondary or tertiary.
- Let's start with neopentyl alcohol. Its structure is given below

As we can see here, the carbon atom holding the OH group is attached directly to only one carbon atom. Hence, it’s a primary alcohol.
- Isopropyl alcohol has the following structure

In isopropyl alcohol, the carbon that is attached with OH group is attached to two carbon atoms and thus it’s a secondary alcohol.
- Carbinol is another name for methanol ($C{{H}_{3}}OH$)

Even though there are no alkyl groups directly linked to the carbon atom, it's also considered as primary alcohol.
- The only remaining option is 2-methylbutan-2-ol. Its structure is given as

Here, the carbon atom holding the OH group is attached directly to three alkyl groups. Thus 2-methylbutan-2-ol is a tertiary alcohol.
Therefore, the answer is option (D) 2-methylbutan-2-ol .
Note:
The type of alcohol can also be determined by counting the number of hydrogen atoms bonded to the carbon with the OH group. Primary alcohols have a general formula of R$C{{H}_{2}}OH$, secondary alcohols have ${{R}_{2}}CHOH$ and tertiary alcohols have ${{R}_{3}}COH$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




