
Which of the following is a vinyl group?
(A) \[\left( {{\text{C}}{{\text{H}}_3}} \right){\text{CH}} - \]
(B) \[{\text{HC}} \equiv {\text{C}} - \]
(C) \[{{\text{H}}_2}{\text{C}} = {\text{CH}} - {\text{C}}{{\text{H}}_2} - \]
(D) \[{\text{C}}{{\text{H}}_2} = {\text{CH}} - \]
Answer
233.1k+ views
Hint: We know that unsaturated hydrocarbons in which carbon atoms are bound to each other with double bonds are called alkenes. If one hydrogen atom from such compounds is removed, we can get a totally different compound.
Complete step by step answer:
Vinyl groups are also called ethenyl. It is basically ethylene with one less hydrogen atom less.
So, if we remove 1 Hydrogen from ethylene we get,
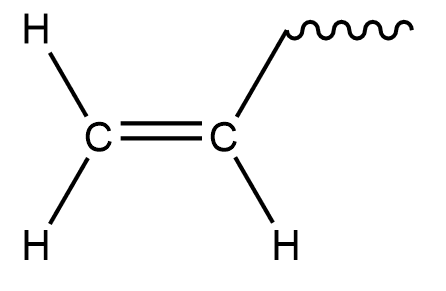
Hence \[{\text{C}}{{\text{H}}_2} = {\text{CH}} - \]is a vinyl group.
So, the correct option is D.
Additional information: Vinyl groups need to be activated by some other group for enhanced reactivity. Activation increases the polarity of the bond and hence also increases reactivity. Vinyl groups are often found as intermediates in preparation of various organic compounds. In hydrohalogenation of alkynes i.e. (addition of hydrogen and halogen) vinyl groups containing intermediates are formed. For example –
\[\begin{gathered}
{\text{H}} - {\text{C}} \equiv {\text{C}} - {\text{H + H}} - {\text{Br }} \to {\text{ [C}}{{\text{H}}_2} = {\text{CH}} - {\text{Br] }} \to {\text{ C}}{{\text{H}}_3} - {\text{CH}} - {\text{B}}{{\text{r}}_2} \\
{\text{ethyne vinyl bromide dibromo ethane}} \\
\end{gathered} \]
In the above reaction, when added to ethylene, vinyl intermediate is formed. Which on further reaction gives dibromo ethane. This reaction is used in preparation of secondary alkyl halides.
Vinyl groups are known to polymerize faster when a catalyst is used. Polymers formed are called vinyl polymers. However, these polymers do not contain any vinyl groups.
On industrial level manufacturing of polyvinyl chloride, vinyl chloride is used as a starting compound which contains vinyl groups.
Note: Out of the given four options, A is a tertiary butyl group and C option is an allylic group. Allylic group is often confused with vinyl group. We can remember it in a way that vinyl is formed when ethylene loses one hydrogen atom.
Complete step by step answer:
Vinyl groups are also called ethenyl. It is basically ethylene with one less hydrogen atom less.
So, if we remove 1 Hydrogen from ethylene we get,

Hence \[{\text{C}}{{\text{H}}_2} = {\text{CH}} - \]is a vinyl group.
So, the correct option is D.
Additional information: Vinyl groups need to be activated by some other group for enhanced reactivity. Activation increases the polarity of the bond and hence also increases reactivity. Vinyl groups are often found as intermediates in preparation of various organic compounds. In hydrohalogenation of alkynes i.e. (addition of hydrogen and halogen) vinyl groups containing intermediates are formed. For example –
\[\begin{gathered}
{\text{H}} - {\text{C}} \equiv {\text{C}} - {\text{H + H}} - {\text{Br }} \to {\text{ [C}}{{\text{H}}_2} = {\text{CH}} - {\text{Br] }} \to {\text{ C}}{{\text{H}}_3} - {\text{CH}} - {\text{B}}{{\text{r}}_2} \\
{\text{ethyne vinyl bromide dibromo ethane}} \\
\end{gathered} \]
In the above reaction, when added to ethylene, vinyl intermediate is formed. Which on further reaction gives dibromo ethane. This reaction is used in preparation of secondary alkyl halides.
Vinyl groups are known to polymerize faster when a catalyst is used. Polymers formed are called vinyl polymers. However, these polymers do not contain any vinyl groups.
On industrial level manufacturing of polyvinyl chloride, vinyl chloride is used as a starting compound which contains vinyl groups.
Note: Out of the given four options, A is a tertiary butyl group and C option is an allylic group. Allylic group is often confused with vinyl group. We can remember it in a way that vinyl is formed when ethylene loses one hydrogen atom.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




