
Which of the following is an example of a cyclic ketal?
A.
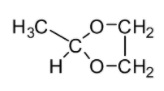
B.
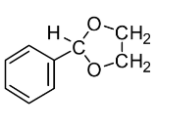
C.
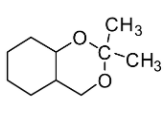
D.
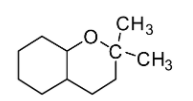




Answer
569.4k+ views
Hint: In organic chemistry, a ketal refers to a functional group that has been derived from a ketone with the replacement of a carbonyl (\[C = O\]) group by two alkoxy groups. In other words, a ketal can also be referred to as an acetal derived from the ketone. Therefore, a cyclic ketal is actually a ketal in which ketal carbon along with one or both oxygen atoms are members of a ring.
Complete answer:
Cyclic ketals are generally formed when the reaction between the two molecules i.e. a ketone and a diol takes place. This reaction results into the formation of two main products i.e. an ketal and water as shown below:
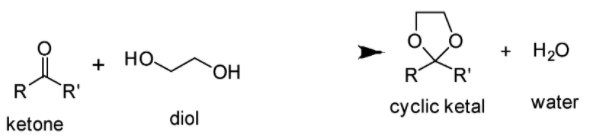
If you look at the structures given in the options, you will notice that none of the structures except mentioned in Option C comprises the ketal carbon (carbon atom having two oxygen atoms) along with oxygen atoms (one or two) which are part of the ring. The reaction of cyclohexane-1,2-diol with acetone leads to the formation of cyclic ketal as demonstrated below:
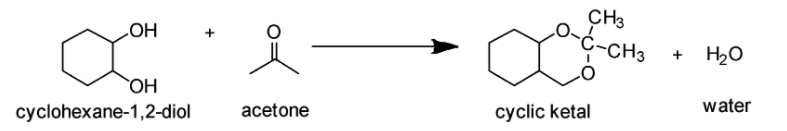
Therefore, the correct answer is Option C.
Note:
Cyclic ketals or cyclic acetals are more stable in comparison to the acetals because of the presence of chelate effect that is majorly derived from having both of the\[ - OH\] groups of acetal being connected to each other in diol. Cyclic ketals have their major applications in carbohydrate chemistry.
Complete answer:
Cyclic ketals are generally formed when the reaction between the two molecules i.e. a ketone and a diol takes place. This reaction results into the formation of two main products i.e. an ketal and water as shown below:

If you look at the structures given in the options, you will notice that none of the structures except mentioned in Option C comprises the ketal carbon (carbon atom having two oxygen atoms) along with oxygen atoms (one or two) which are part of the ring. The reaction of cyclohexane-1,2-diol with acetone leads to the formation of cyclic ketal as demonstrated below:

Therefore, the correct answer is Option C.
Note:
Cyclic ketals or cyclic acetals are more stable in comparison to the acetals because of the presence of chelate effect that is majorly derived from having both of the\[ - OH\] groups of acetal being connected to each other in diol. Cyclic ketals have their major applications in carbohydrate chemistry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




