
Which of the following is an example of a super octet molecule:
A. $SF_{ 6 }$
B. $PCl_{ 5 }$
C. $IF_{ 7 }$
D. All three
Answer
604.2k+ views
Hint: To answer this question you must know about super octet molecules. A super octet molecule is the one that has more than 8 electrons in an ultimate(valence) shell around a central metal atom. Now try to use this concept for each molecule given in the options.
Complete step by step answer:
As we already know a super octet molecule is the one that has more than 8 electrons in an ultimate(valence) shell around a central metal atom.
Now, let’s look at all the molecules one by one,
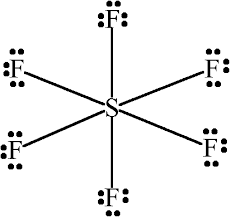
$SF_{ 6 }$, the central atom S shares 6 of its electrons with 6 electrons, one each from 6 fluorine atoms making the total electrons of the outermost shell of the central atom Sulfur to 12 electrons.
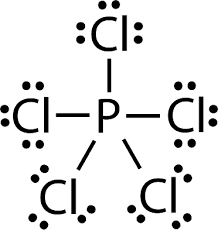
$PCl_{ 5 }$, the central atom P shares 5 of its electrons with 5 electrons, one each from 5 chlorine atoms making the total electrons of the outermost shell of the central atom Phosphorus to 10 electrons.
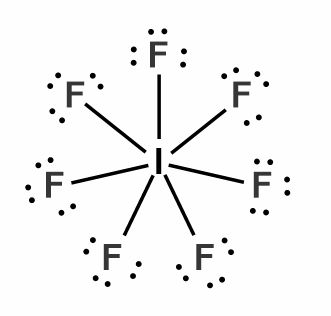
$IF_{ 7 }$, Iodine in its outermost shell shares 7 of its electrons with one electron from 7 fluorine atoms. The total no of electrons in the outermost shell of the central atom is 14.
We can see that all of them have more than 8 electrons in their ultimate shell around a central metal atom.
Therefore, we can conclude that the correct answer to this question is option D.
Note: We should also know about the octet rule. The octet rule states that atoms below atomic number 20 tend to combine so that they each have eight electrons in their valence shells, which gives them the same electronic configuration as a noble gas.
The rule is applicable to the main- group elements, especially carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium and magnesium.
Complete step by step answer:
As we already know a super octet molecule is the one that has more than 8 electrons in an ultimate(valence) shell around a central metal atom.
Now, let’s look at all the molecules one by one,
$SF_{ 6 }$, the central atom S shares 6 of its electrons with 6 electrons, one each from 6 fluorine atoms making the total electrons of the outermost shell of the central atom Sulfur to 12 electrons.

$PCl_{ 5 }$, the central atom P shares 5 of its electrons with 5 electrons, one each from 5 chlorine atoms making the total electrons of the outermost shell of the central atom Phosphorus to 10 electrons.

$IF_{ 7 }$, Iodine in its outermost shell shares 7 of its electrons with one electron from 7 fluorine atoms. The total no of electrons in the outermost shell of the central atom is 14.

We can see that all of them have more than 8 electrons in their ultimate shell around a central metal atom.
Therefore, we can conclude that the correct answer to this question is option D.
Note: We should also know about the octet rule. The octet rule states that atoms below atomic number 20 tend to combine so that they each have eight electrons in their valence shells, which gives them the same electronic configuration as a noble gas.
The rule is applicable to the main- group elements, especially carbon, nitrogen, oxygen, and the halogens, but also to metals such as sodium and magnesium.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




