
Which of the following is an example of non-biodegradable detergent?
A:
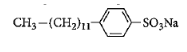
B:
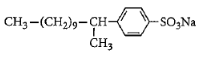
C:
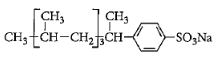
D: $C{H_3} - {\left( {C{H_2}} \right)_{10}} - C{H_2} - OS{O_2}Na$
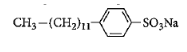


Answer
594k+ views
Hint:Biodegradable substances are the substances which can be decomposed by the action of microorganisms. Non-biodegradable substances are the substances which are not decomposed by the action of microorganisms.
Complete answer:
Surfactants are the compounds that lower the surface tension between two liquids, gas, and a liquid or between a liquid and a solid. Surface tension is defined as the property of the surface of a liquid that allows it to resist an external force. It is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid. Surfactants may act as detergents, emulsifiers, foaming agents. Detergent is a surfactant or mixture of surfactants that contain cleansing properties. It is formed from a long chain alkyl group. General formula of detergents is $RSO_4^ - N{a^ + }$. Like soap, these contain both hydrophilic parts and hydrophobic parts. More number of branches will require more energy for dissociation. So detergent which will contain the maximum number of branches will be non-biodegradable. Among given options, detergent in option C has a maximum number of branches. So answer to this question is option C that is:
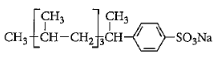
Answer to this question is option C.
Additional information:} Soap is basically sodium and potassium salt of long-chain fatty acid $RCO{O^ - }N{a^ + }$ . The end of the molecule to which the sodium ion is attached is polar in nature that means it will dissolve in water as water is also polar in nature. As it is dissolved in water it is called the hydrophilic part means water loving. The alkyl chain is nonpolar hence it does not dissolve in water. Due to this reason, it is called hydrophobic part which means water-hating.
Note:
Branched alkyl groups do not dissociate easily as it becomes difficult to break a number of bonds. Detergents are basically long-chain alkyl groups. So basically compounds with more branches will be difficult to dissociate and will be non-biodegradable.
Complete answer:
Surfactants are the compounds that lower the surface tension between two liquids, gas, and a liquid or between a liquid and a solid. Surface tension is defined as the property of the surface of a liquid that allows it to resist an external force. It is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid. Surfactants may act as detergents, emulsifiers, foaming agents. Detergent is a surfactant or mixture of surfactants that contain cleansing properties. It is formed from a long chain alkyl group. General formula of detergents is $RSO_4^ - N{a^ + }$. Like soap, these contain both hydrophilic parts and hydrophobic parts. More number of branches will require more energy for dissociation. So detergent which will contain the maximum number of branches will be non-biodegradable. Among given options, detergent in option C has a maximum number of branches. So answer to this question is option C that is:

Answer to this question is option C.
Additional information:} Soap is basically sodium and potassium salt of long-chain fatty acid $RCO{O^ - }N{a^ + }$ . The end of the molecule to which the sodium ion is attached is polar in nature that means it will dissolve in water as water is also polar in nature. As it is dissolved in water it is called the hydrophilic part means water loving. The alkyl chain is nonpolar hence it does not dissolve in water. Due to this reason, it is called hydrophobic part which means water-hating.
Note:
Branched alkyl groups do not dissociate easily as it becomes difficult to break a number of bonds. Detergents are basically long-chain alkyl groups. So basically compounds with more branches will be difficult to dissociate and will be non-biodegradable.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




