
Which of the following is condensation polymer?
A.Polypropylene
B.PMMA
C.Glyptal
D.Teflon
Answer
578.4k+ views
Hint: Polymers are high molecular mass substances where every molecule is derived from a large number of simple molecules joined together. They are formed from repeated combinations of simplest units known as monomers and the process of formation of polymers is called polymerization. Condensation polymers are polymers in which molecules of monomer combine with elimination of simple molecules like water, alcohol or ammonia.
Complete step by step answer:
Polypropylene is an additional polymer. It is formed by the polymerization of monomer propylene which has a carbon-carbon double bond.
The carbon-carbon double bond breaks during polymerization and n-molecules of the same monomer are attached on both sides of the chain.
The reaction is given as-
$n{\text{ C}}{{\text{H}}_2} = {\text{CHC}}{{\text{H}}_{\text{3}}}\xrightarrow{{Polymerization}}{\left[ { - CH\left( {C{H_3}} \right) - C{H_2} - } \right]_n}$
PMMA (Polymethylmethacrylate) is an additional polymer. It is formed by the polymerization of monomer methyl methacrylate which has a carbon-carbon double bond.
There is no elimination of any molecule in this reaction.
The reaction is given as-$n{\text{ C}}{{\text{H}}_2} = {\text{CH}}\left( {{\text{COOC}}{{\text{H}}_{\text{3}}}} \right)\xrightarrow{{Polymerization}}{\left[ { - {\text{C}}{{\text{H}}_2} - {\text{CH}}\left( {{\text{COOC}}{{\text{H}}_3}} \right) - } \right]_n}$
It is also called Plexiglas or Perspex.
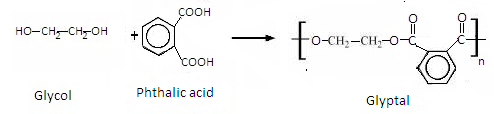
Glycol is a condensation polymer. It is polyester formed by the reaction of Phthalic acid and ethylene glycol. Water molecules get eliminated in this reaction.
The reaction is given as-
Teflon is an additional polymer. It is prepared by polymerization of monomer tetrafluoroethylene which has a carbon-carbon double bond.
Tetrafluoroethylene has four fluorine atoms in which two fluorines are attached to carbon atoms. The double bond breaks during polymerization.
The reaction is as follows-
$n{\text{ }}\mathop {{\text{C}}{{\text{F}}_2} = {\text{C}}{{\text{F}}_2}}\limits_{{\text{Tetrafluoroethylene}}} \xrightarrow{{{\text{Polymerisation}}}}\mathop {{{\left[ { - {\text{C}}{{\text{F}}_2} - {\text{C}}{{\text{F}}_2} - } \right]}_n}}\limits_{{\text{Teflon}}} $
It is also known as PTFE because its IUPAC name is Poly Tetrafluoroethylene.
Hence the correct answer is C.
Note:
The uses of the given polymers are-
-Polypropylene is used in making carpet, upholstery and in packaging.
-PMMA is used in lighting covers, signs and skylights.
-Glyptal is used in paints and lacquers.
-Teflon is used for non-stick coating for cooking utensils like cooking pans and in curling irons.
Complete step by step answer:
Polypropylene is an additional polymer. It is formed by the polymerization of monomer propylene which has a carbon-carbon double bond.
The carbon-carbon double bond breaks during polymerization and n-molecules of the same monomer are attached on both sides of the chain.
The reaction is given as-
$n{\text{ C}}{{\text{H}}_2} = {\text{CHC}}{{\text{H}}_{\text{3}}}\xrightarrow{{Polymerization}}{\left[ { - CH\left( {C{H_3}} \right) - C{H_2} - } \right]_n}$
PMMA (Polymethylmethacrylate) is an additional polymer. It is formed by the polymerization of monomer methyl methacrylate which has a carbon-carbon double bond.
There is no elimination of any molecule in this reaction.
The reaction is given as-$n{\text{ C}}{{\text{H}}_2} = {\text{CH}}\left( {{\text{COOC}}{{\text{H}}_{\text{3}}}} \right)\xrightarrow{{Polymerization}}{\left[ { - {\text{C}}{{\text{H}}_2} - {\text{CH}}\left( {{\text{COOC}}{{\text{H}}_3}} \right) - } \right]_n}$
It is also called Plexiglas or Perspex.
Glycol is a condensation polymer. It is polyester formed by the reaction of Phthalic acid and ethylene glycol. Water molecules get eliminated in this reaction.
The reaction is given as-

Teflon is an additional polymer. It is prepared by polymerization of monomer tetrafluoroethylene which has a carbon-carbon double bond.
Tetrafluoroethylene has four fluorine atoms in which two fluorines are attached to carbon atoms. The double bond breaks during polymerization.
The reaction is as follows-
$n{\text{ }}\mathop {{\text{C}}{{\text{F}}_2} = {\text{C}}{{\text{F}}_2}}\limits_{{\text{Tetrafluoroethylene}}} \xrightarrow{{{\text{Polymerisation}}}}\mathop {{{\left[ { - {\text{C}}{{\text{F}}_2} - {\text{C}}{{\text{F}}_2} - } \right]}_n}}\limits_{{\text{Teflon}}} $
It is also known as PTFE because its IUPAC name is Poly Tetrafluoroethylene.
Hence the correct answer is C.
Note:
The uses of the given polymers are-
-Polypropylene is used in making carpet, upholstery and in packaging.
-PMMA is used in lighting covers, signs and skylights.
-Glyptal is used in paints and lacquers.
-Teflon is used for non-stick coating for cooking utensils like cooking pans and in curling irons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




