
Which of the following is correct with respect to the acidity of benzene sulphonic acid and benzoic acid?
A. Benzene sulphonic acid >Benzoic acid
B. Benzene sulphonic acid <Benzoic acid
C. Benzene sulphonic acid =Benzoic acid
D. None of the above
Answer
577.5k+ views
Hint: The strength of an acid depends on the ability of acid to donate the proton and stability of the conjugate base. Acid having more number of resonance structures is a stronger acid.
Step by step answer: Draw the structures of benzoic acid and its conjugate base.
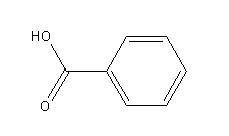
-Benzoic acid
Benzoic acid donates its proton and forms benzoate ion as a conjugate base.
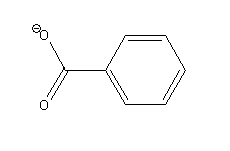
-Benzoate ion
The possible resonance structures of benzoate ion are as follows:
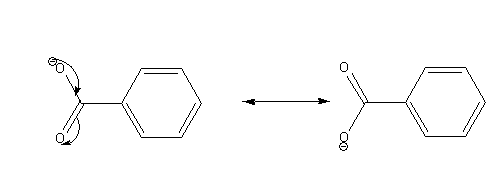
Draw the structures of benzene sulphonic acid and its conjugate base.
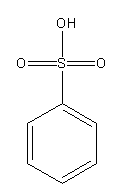
Benzene sulphonic acid
Benzene sulphonic acid donates its proton and forms benzene sulfonate ion as a conjugate base.

Benzene sulfonate ion
The possible resonance structures of benzene sulfonate ion are as follows:

The conjugate base of an acid having more number of resonance structures is more stable. In this case, benzene sulfonate ion is more stable than benzoate ion as it shows more number of resonance structures than the benzoate ion. Thus benzene sulphonic acid is the stronger acid than benzoic acid.
Hence, the decrease in order of strength of given acids is:
Benzene sulphonic acid >Benzoic acid
Thus, the correct option is (A) Benzene sulphonic acid >Benzoic acid
Note: The strength of an acid depends on how easily it donates the protons and the stability of the conjugate base. Delocalization of negative charge in resonance stabilizes the conjugate base. So, the more the number of resonance structures, the more stable is the conjugate base and the stronger is the acid.
Step by step answer: Draw the structures of benzoic acid and its conjugate base.

-Benzoic acid
Benzoic acid donates its proton and forms benzoate ion as a conjugate base.

-Benzoate ion
The possible resonance structures of benzoate ion are as follows:

Draw the structures of benzene sulphonic acid and its conjugate base.

Benzene sulphonic acid
Benzene sulphonic acid donates its proton and forms benzene sulfonate ion as a conjugate base.

Benzene sulfonate ion
The possible resonance structures of benzene sulfonate ion are as follows:

The conjugate base of an acid having more number of resonance structures is more stable. In this case, benzene sulfonate ion is more stable than benzoate ion as it shows more number of resonance structures than the benzoate ion. Thus benzene sulphonic acid is the stronger acid than benzoic acid.
Hence, the decrease in order of strength of given acids is:
Benzene sulphonic acid >Benzoic acid
Thus, the correct option is (A) Benzene sulphonic acid >Benzoic acid
Note: The strength of an acid depends on how easily it donates the protons and the stability of the conjugate base. Delocalization of negative charge in resonance stabilizes the conjugate base. So, the more the number of resonance structures, the more stable is the conjugate base and the stronger is the acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




