
Which of the following is isooctane?
A.
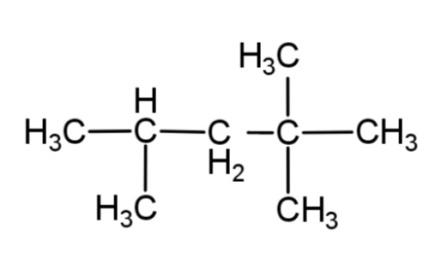
B.
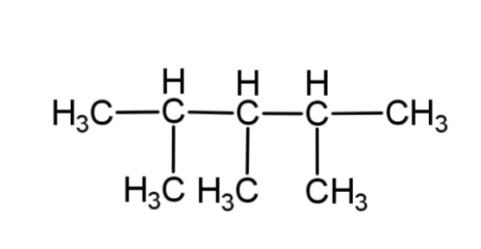
C.
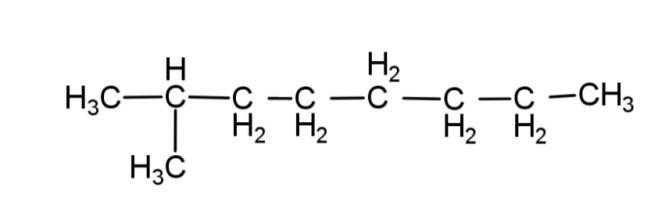
D. None



Answer
528.9k+ views
Hint: Alkanes are the hydrocarbons having only single bonds. They are named according to the number of carbon atoms present in the molecules. Iso is a prefix used for naming branched alkyl chains where one methyl group is attached as a branch on the carbon of straight chain.
Complete answer:
The naming of organic compounds is done by following the norms and standards given by IUPAC. According to IUPAC, some of the nomenclature rules followed in the given compounds with straight or branched chain carbon atoms are:
- the longest possible carbon chain is selected for naming, this is called the parent chain. The carbon atoms that are not included in this chain are called branched chain or side chain.
- this longest chain can be zig – zag or a straight chain.
- the numbering is done as the carbon having branches gets the lowest possible number.
- for alkyl chains the prefix ‘iso’ is used for a branch of 1 alkyl group on the carbon atom. This iso prefix is the fundamental name and written alphabetically.
- alkenes are named according to the number of carbon atoms present, like 1 carbon – methane, 2 carbon – ethane etc.
From these rules, the structure of isooctane, will have 8 – carbon atoms, as octane has 8 carbons, and along with this it will have only 1 methyl group as a branch. So the structure of isooctane is:
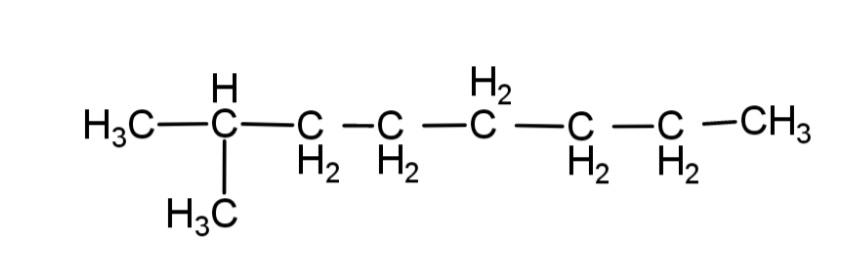
Hence, option C is correct.
Note:
Iso is given as a prefix with only 1 methyl branch, while neo prefix is given for methyl branches in consecutive adjacent carbon atoms. These are the prefix for simple alkyl branches, while ‘sec’ and ‘tert’ prefix are given to the alkyl branches that have functional groups, or secondary carbons.
Complete answer:
The naming of organic compounds is done by following the norms and standards given by IUPAC. According to IUPAC, some of the nomenclature rules followed in the given compounds with straight or branched chain carbon atoms are:
- the longest possible carbon chain is selected for naming, this is called the parent chain. The carbon atoms that are not included in this chain are called branched chain or side chain.
- this longest chain can be zig – zag or a straight chain.
- the numbering is done as the carbon having branches gets the lowest possible number.
- for alkyl chains the prefix ‘iso’ is used for a branch of 1 alkyl group on the carbon atom. This iso prefix is the fundamental name and written alphabetically.
- alkenes are named according to the number of carbon atoms present, like 1 carbon – methane, 2 carbon – ethane etc.
From these rules, the structure of isooctane, will have 8 – carbon atoms, as octane has 8 carbons, and along with this it will have only 1 methyl group as a branch. So the structure of isooctane is:

Hence, option C is correct.
Note:
Iso is given as a prefix with only 1 methyl branch, while neo prefix is given for methyl branches in consecutive adjacent carbon atoms. These are the prefix for simple alkyl branches, while ‘sec’ and ‘tert’ prefix are given to the alkyl branches that have functional groups, or secondary carbons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




