
Which of the following is most polar?
(A)
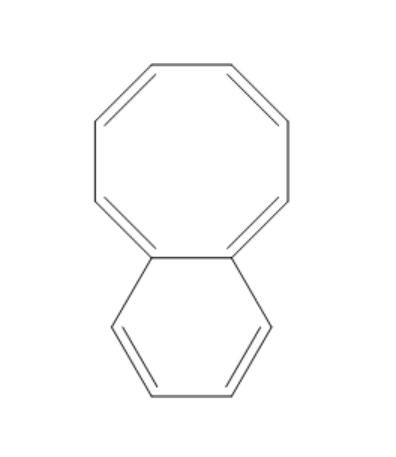
(B)
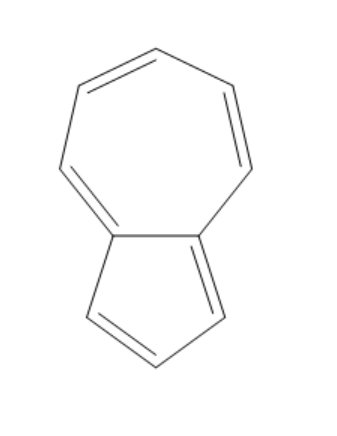
(C)
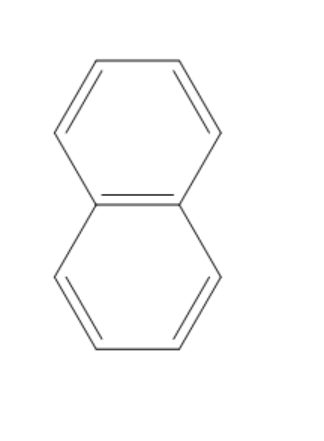
(D)
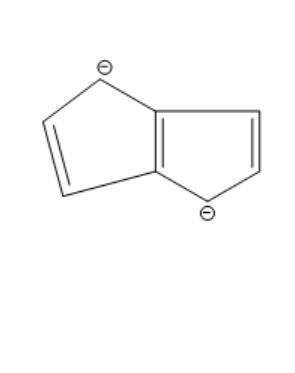




Answer
521.1k+ views
Hint : To find which compound is more polar, check whether positive or negative is generated or not. If positive or negative charge is generated, check the stability of the compound. If the ring is stable after gaining charge, then that compound is said to be more stable.
Complete Step By Step Answer:
The compound given in the first option has $ 12\pi {e^ - } $ and it is antiaromatic. If we break a bond, we get a compound with charges. Now, we can observe that the compound is not aromatic and because of this reason it is not most polar. If we break a bond in the compound given in the second option, we get a compound with charges and all the rings in the compound are aromatic. It is said to be a quasi-aromatic compound. So, it is the most stable compound. We cannot break any bond in the third compound, because both the rings are aromatic and they are stable. So, polarity cannot be generated in this compound. The compound in the fourth option has two aromatic rings. So, this compound also will not allow the breaking of bonds as they are already stable. So, polarity cannot be generated in this compound also.
Therefore, option B is the correct answer.
Note :
Aromaticity describes a conjugated system having alternating single and double bonds in a ring. This configuration allows the electrons in the molecule pi system to be delocalized around the ring which increases the stability of the molecule.
Complete Step By Step Answer:
The compound given in the first option has $ 12\pi {e^ - } $ and it is antiaromatic. If we break a bond, we get a compound with charges. Now, we can observe that the compound is not aromatic and because of this reason it is not most polar. If we break a bond in the compound given in the second option, we get a compound with charges and all the rings in the compound are aromatic. It is said to be a quasi-aromatic compound. So, it is the most stable compound. We cannot break any bond in the third compound, because both the rings are aromatic and they are stable. So, polarity cannot be generated in this compound. The compound in the fourth option has two aromatic rings. So, this compound also will not allow the breaking of bonds as they are already stable. So, polarity cannot be generated in this compound also.
Therefore, option B is the correct answer.
Note :
Aromaticity describes a conjugated system having alternating single and double bonds in a ring. This configuration allows the electrons in the molecule pi system to be delocalized around the ring which increases the stability of the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




