
Which of the following is not a \[\pi - bonded\] system?
A.Zeise’s salt
B.Ferrocene
C.Dibenzene Chromium
D.Tetraethyl lead
Answer
509.7k+ views
Hint: A \[\pi - bonded\] system must contain at least one double or triple bond in the complex. Aromatic systems, alkenes or alkynes as ligands present independently or as part of organometallic complexes contribute \[\pi - electrons\] present in their unhybridized p-orbitals.
Complete answer:
In order to determine whether a system contains \[\pi - electrons\] or not, the complete structure of the compound must be known.
1.Zeise’s salt : It is an organometallic compound containing platinum as the central metal. Platinum is bonded to three chloride ligands and one ethene ligand with an overall negative charge on the complex.
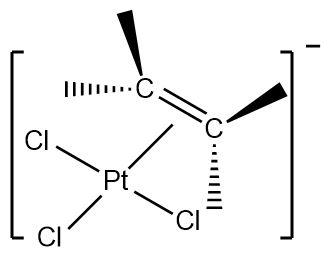
Hence, the given complex is a \[\pi - bonded\] system.
2.Ferrocene: It is an organometallic compound also known as a sandwiched compound as it contains an iron atom sandwiched between two cyclopentadienyl rings. Cyclopentadienyl rings are aromatic in nature containing two double bonds and a lone pair (a total of 6 \[\pi - electrons\]) that are delocalized throughout the ring.

Hence, Ferrocene is a \[\pi - bonded\] system.
3.Dibenzene chromium: It is also an organometallic compound containing chromium metal sandwiched between two benzene rings. Here benzene rings act as ligands with hapticity six. Benzene in itself is an aromatic compound and contains six \[\pi - electrons\] each.
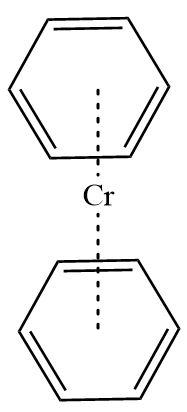
Hence, Dibenzene chromium is a \[\pi - bonded\]
4.Tetraethyl lead: An organometallic compound containing four ethyl groups attached to a lead atom. The ethyl groups are completely saturated and do not contain any \[\pi - electrons\].
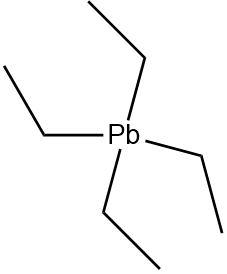
Due to the lack of double, triple bonds or aromatic systems Tetraethyl lead is not a \[\pi - bonded\] system.
Thus, option (D) Tetraethyl lead is not a \[\pi - bonded\] system.
Note:
Many organometallic compounds contain organic compounds as hapto ligands. Hapticity is the measure of the number of carbon atoms that are directly bonded to the central metal while donating its electrons.
Complete answer:
In order to determine whether a system contains \[\pi - electrons\] or not, the complete structure of the compound must be known.
1.Zeise’s salt : It is an organometallic compound containing platinum as the central metal. Platinum is bonded to three chloride ligands and one ethene ligand with an overall negative charge on the complex.

Hence, the given complex is a \[\pi - bonded\] system.
2.Ferrocene: It is an organometallic compound also known as a sandwiched compound as it contains an iron atom sandwiched between two cyclopentadienyl rings. Cyclopentadienyl rings are aromatic in nature containing two double bonds and a lone pair (a total of 6 \[\pi - electrons\]) that are delocalized throughout the ring.

Hence, Ferrocene is a \[\pi - bonded\] system.
3.Dibenzene chromium: It is also an organometallic compound containing chromium metal sandwiched between two benzene rings. Here benzene rings act as ligands with hapticity six. Benzene in itself is an aromatic compound and contains six \[\pi - electrons\] each.

Hence, Dibenzene chromium is a \[\pi - bonded\]
4.Tetraethyl lead: An organometallic compound containing four ethyl groups attached to a lead atom. The ethyl groups are completely saturated and do not contain any \[\pi - electrons\].

Due to the lack of double, triple bonds or aromatic systems Tetraethyl lead is not a \[\pi - bonded\] system.
Thus, option (D) Tetraethyl lead is not a \[\pi - bonded\] system.
Note:
Many organometallic compounds contain organic compounds as hapto ligands. Hapticity is the measure of the number of carbon atoms that are directly bonded to the central metal while donating its electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




