
Which of the following is not a redox change?
A. $2{H_2}S + S{O_2} \to 2{H_2}O + 3S$
B. $2BaO + {O_2} \to 2Ba{O_2}$
C. $Ba{O_2} + {H_2}S{O_4} \to BaS{O_4} + {H_2}{O_2}$
D. $2KCl{O_3} \to 2KCl + 3{O_2}$
Answer
561.3k+ views
Hint: We know that redox is a short form for reduction and oxidation. In redox reaction one species is reduced while the other is oxidized simultaneously. If there will be a change in the oxidation number of the reactants and the products then the reaction will be called a redox or disproportionate reaction.
Complete answer:
A redox reaction is a chemical reaction in which the oxidation number of a molecule, atom or ion changes by gaining or losing an electron.
Here we need to find out which of the following reactions is not undergoing oxidation and reduction and for that we need to find out the oxidation states of the reactants and the products.
A. $2{H_2}S + S{O_2} \to 2{H_2}O + 3S$
Here in this reaction the oxidation number of different species is changing so it will undergo oxidation and reduction and hence this will be a redox reaction.
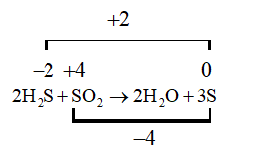
B. $2BaO + {O_2} \to 2Ba{O_2}$
This reaction is also undergoing reduction and oxidation as the oxidation states of the reactants and the products are changing hence this will also be a redox reaction.
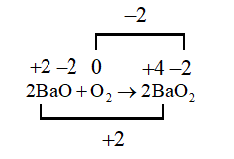
C. $Ba{O_2} + {H_2}S{O_4} \to BaS{O_4} + {H_2}{O_2}$
This reaction is a double decomposition and hence is not a redox reaction as it does show a change in the oxidation state and therefore the species do not undergo oxidation and reduction.
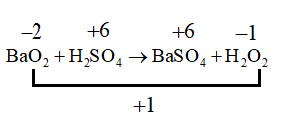
Here we know that only reduction is taking place.
D. $2KCl{O_3} \to 2KCl + 3{O_2}$
In this reaction both oxidation and reduction are taking place hence this is a redox reaction
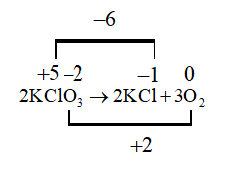
Therefore the correct answer is ‘C. $Ba{O_2} + {H_2}S{O_4} \to BaS{O_4} + {H_2}{O_2}$’.
Note: The species which is being reduced acts as an oxidizing agent. On the other hand the species undergoing oxidation will act as a reducing agent. A Double decomposition reaction is the one in which each compound is interchanged in order to form two new compounds. Compounds where the positive ion of one compound is exchanged with another is called double replacement reaction.
Complete answer:
A redox reaction is a chemical reaction in which the oxidation number of a molecule, atom or ion changes by gaining or losing an electron.
Here we need to find out which of the following reactions is not undergoing oxidation and reduction and for that we need to find out the oxidation states of the reactants and the products.
A. $2{H_2}S + S{O_2} \to 2{H_2}O + 3S$
Here in this reaction the oxidation number of different species is changing so it will undergo oxidation and reduction and hence this will be a redox reaction.
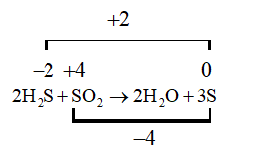
B. $2BaO + {O_2} \to 2Ba{O_2}$
This reaction is also undergoing reduction and oxidation as the oxidation states of the reactants and the products are changing hence this will also be a redox reaction.
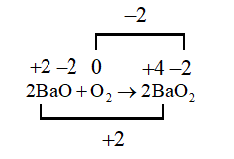
C. $Ba{O_2} + {H_2}S{O_4} \to BaS{O_4} + {H_2}{O_2}$
This reaction is a double decomposition and hence is not a redox reaction as it does show a change in the oxidation state and therefore the species do not undergo oxidation and reduction.
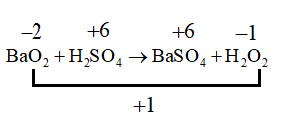
Here we know that only reduction is taking place.
D. $2KCl{O_3} \to 2KCl + 3{O_2}$
In this reaction both oxidation and reduction are taking place hence this is a redox reaction
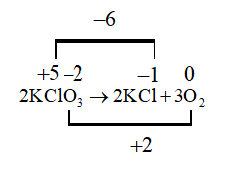
Therefore the correct answer is ‘C. $Ba{O_2} + {H_2}S{O_4} \to BaS{O_4} + {H_2}{O_2}$’.
Note: The species which is being reduced acts as an oxidizing agent. On the other hand the species undergoing oxidation will act as a reducing agent. A Double decomposition reaction is the one in which each compound is interchanged in order to form two new compounds. Compounds where the positive ion of one compound is exchanged with another is called double replacement reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




