
Which of the following is not chiral?
A.2-Butanol
B.2, 3-Dibromopentane
C.3-Bromopentane
D.2-Hydroxypropanoic acid
Answer
497.1k+ views
Hint: To check whether a compound is chiral or not, we have to look at the structure of the compound. So, in this question we have to check the structure of each option to know which of the following options is not chiral.
Complete answer:
To check the chirality of any compound, we first have to know what chirality is. Chirality is a property of a compound which shows that the compound cannot form a mirror image of itself. We can also say that if a compound is chiral, it has a non-superimposable molecule.
As chirality of a substance deals with the mirror image of a compound, we have to check the chirality of each option by looking at its structure.
The compound given in option A, is 2-Butanol. The structure of 2-Butanol is given below.
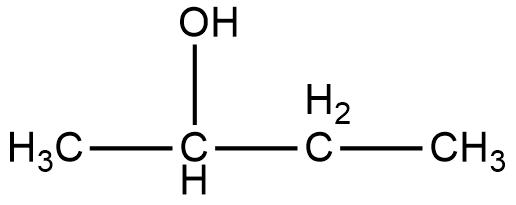
As it can be seen in the figure, the alcohol group in the compound 2-Butanol is attached to the second carbon, and that is why it is named as 2-Butanol. Now, if we look at the mirror image of this compound, we can see that the alcohol group is attached to the third carbon of the compound.
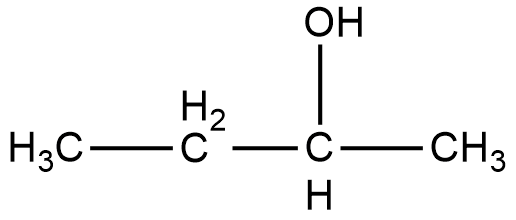
Therefore, if we try to superimpose both the images, we cannot get the same compound. Thus, 2-Butanol is a chiral compound.
Now, the compound given in option B is 2, 3-Dibromopentane. The structure of 2, 3-Dibromopentane is shown below.
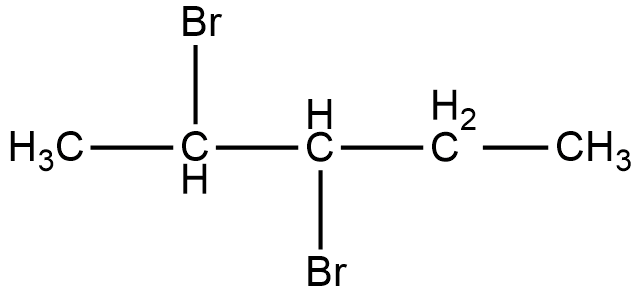
As it can be seen in the figure, two bromine atoms are attached to the second and third carbon from the left hand side of the chain. Now, if we look at the mirror image of this compound, we can see that the two bromine atoms shift to third and fourth carbon atoms from the left hand side of the chain.
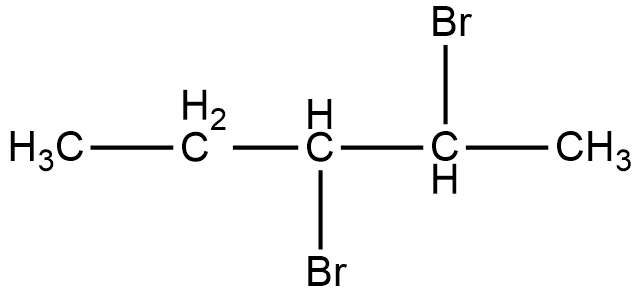
Therefore, if we try to superimpose both the images, we cannot get the same compound. Thus, 2, 3-dibromopentane is a chiral compound.
Now, the compound given in option C is 3-bromopentane. The structure of 3-bromopentane is shown below.
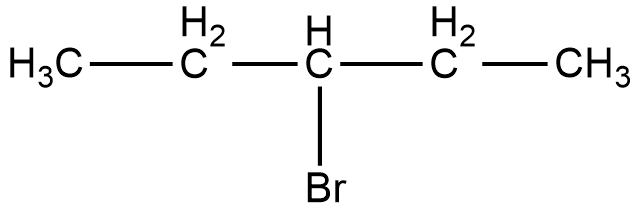
As it can be seen in the figure, the bromine atom is attached to the third carbon from the left hand side or the right hand side of the compound. Now, if we take a look at the mirror image of this compound, we can see that there is no difference in the position of the bromine atom.
Therefore, if we try to superimpose both the images, we will get the same compound. Thus, 3-bromopentane is not a chiral compound.
Therefore, the answer to this question is option C, 3-dibromopentane.
Note:
The compounds which are not chiral are also called achiral compounds. So, we can say that 3-bromopentane is an achiral compound. If we were to pass a plane from the centre of an achiral compound, the substances on the right will be exactly the same as the substances on the left. Thus, chirality of a substance can also be found out like that.
Complete answer:
To check the chirality of any compound, we first have to know what chirality is. Chirality is a property of a compound which shows that the compound cannot form a mirror image of itself. We can also say that if a compound is chiral, it has a non-superimposable molecule.
As chirality of a substance deals with the mirror image of a compound, we have to check the chirality of each option by looking at its structure.
The compound given in option A, is 2-Butanol. The structure of 2-Butanol is given below.

As it can be seen in the figure, the alcohol group in the compound 2-Butanol is attached to the second carbon, and that is why it is named as 2-Butanol. Now, if we look at the mirror image of this compound, we can see that the alcohol group is attached to the third carbon of the compound.

Therefore, if we try to superimpose both the images, we cannot get the same compound. Thus, 2-Butanol is a chiral compound.
Now, the compound given in option B is 2, 3-Dibromopentane. The structure of 2, 3-Dibromopentane is shown below.

As it can be seen in the figure, two bromine atoms are attached to the second and third carbon from the left hand side of the chain. Now, if we look at the mirror image of this compound, we can see that the two bromine atoms shift to third and fourth carbon atoms from the left hand side of the chain.

Therefore, if we try to superimpose both the images, we cannot get the same compound. Thus, 2, 3-dibromopentane is a chiral compound.
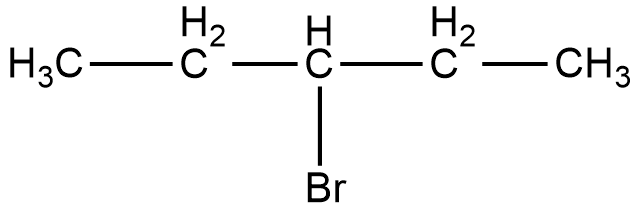
Now, the compound given in option C is 3-bromopentane. The structure of 3-bromopentane is shown below.

As it can be seen in the figure, the bromine atom is attached to the third carbon from the left hand side or the right hand side of the compound. Now, if we take a look at the mirror image of this compound, we can see that there is no difference in the position of the bromine atom.

Therefore, if we try to superimpose both the images, we will get the same compound. Thus, 3-bromopentane is not a chiral compound.
Therefore, the answer to this question is option C, 3-dibromopentane.
Note:
The compounds which are not chiral are also called achiral compounds. So, we can say that 3-bromopentane is an achiral compound. If we were to pass a plane from the centre of an achiral compound, the substances on the right will be exactly the same as the substances on the left. Thus, chirality of a substance can also be found out like that.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




