
Which of the following is not D sugar?
A)
B)
C)
D)




Answer
498.3k+ views
Hint: Carbohydrates are sugar molecules that have asymmetric carbons and are classified into D and L configurations.
D configuration can be given to sugars containing hydroxyl groups right to the farthest asymmetric carbon from the carbonyl group.
L configuration can be given to sugars containing hydroxyl groups left to the farthest asymmetric carbon from the carbonyl group.
Complete answer: Carbohydrates are sugar molecules consisting of mainly carbon, hydrogen and oxygen atoms.
These are also called hydrates of carbon.
The general empirical formula of carbohydrates is \[{C_m}{\left( {{H_2}O} \right)_n}\]
The oxygen and hydrogen are in the ratio of 1:2 in the carbohydrates.
Carbohydrates contain carboxyl and hydroxyl groups.
Based on the hydroxyl group, these sugars are classified into D and L sugars.
The sugar molecule with the asymmetric carbon farther from the carbonyl group contains the hydroxyl group at the left side; it can be called L sugar.
The sugar molecule with the asymmetric carbon farther from the carbonyl group contains the hydroxyl group at the right side; it can be called L sugar.
In the given options,
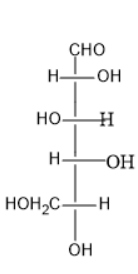
Option (A) contains five carbon atoms; the last carbon is farther from the carbonyl group than the hydroxyl group at the left side.
Thus, it is L sugar.
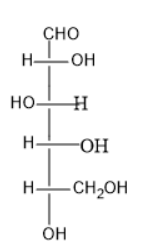
Option (B) contains five carbon atoms; the last carbon is farther from the carbonyl group than the hydroxyl group at right side.
Thus, it is D sugar.
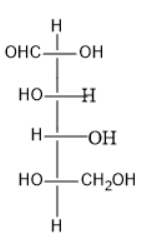
Option (C) contains five carbon atoms; the last carbon is farther from the carbonyl group and contains the hydroxyl group at both right and left side.
Thus, it is neither L nor D sugar.
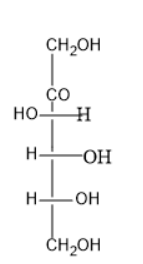
Option (D) contains five carbon atoms; the last carbon is farther from the carbonyl group than the hydroxyl group at right side.
Thus, it is D sugar.
Thus, option A is L-sugar.
Note:
The configuration must be written by considering the farthest carbon from the carbonyl group.
Though the nearest carbons contain hydroxyl groups, they should not be considered.
The direction of the hydroxyl group to the last farther carbon from the carbonyl group must be clearly noticed.
D configuration can be given to sugars containing hydroxyl groups right to the farthest asymmetric carbon from the carbonyl group.
L configuration can be given to sugars containing hydroxyl groups left to the farthest asymmetric carbon from the carbonyl group.
Complete answer: Carbohydrates are sugar molecules consisting of mainly carbon, hydrogen and oxygen atoms.
These are also called hydrates of carbon.
The general empirical formula of carbohydrates is \[{C_m}{\left( {{H_2}O} \right)_n}\]
The oxygen and hydrogen are in the ratio of 1:2 in the carbohydrates.
Carbohydrates contain carboxyl and hydroxyl groups.
Based on the hydroxyl group, these sugars are classified into D and L sugars.
The sugar molecule with the asymmetric carbon farther from the carbonyl group contains the hydroxyl group at the left side; it can be called L sugar.
The sugar molecule with the asymmetric carbon farther from the carbonyl group contains the hydroxyl group at the right side; it can be called L sugar.
In the given options,
Option (A) contains five carbon atoms; the last carbon is farther from the carbonyl group than the hydroxyl group at the left side.
Thus, it is L sugar.
Option (B) contains five carbon atoms; the last carbon is farther from the carbonyl group than the hydroxyl group at right side.
Thus, it is D sugar.
Option (C) contains five carbon atoms; the last carbon is farther from the carbonyl group and contains the hydroxyl group at both right and left side.
Thus, it is neither L nor D sugar.
Option (D) contains five carbon atoms; the last carbon is farther from the carbonyl group than the hydroxyl group at right side.
Thus, it is D sugar.
Thus, option A is L-sugar.
Note:
The configuration must be written by considering the farthest carbon from the carbonyl group.
Though the nearest carbons contain hydroxyl groups, they should not be considered.
The direction of the hydroxyl group to the last farther carbon from the carbonyl group must be clearly noticed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




