
Which of the following is phenyl ethanoate?

Answer
609.6k+ views
Hint- Here, we will proceed by discussing all the organic compounds given in the options of the problem. We will write down the names of these compounds along with basic features of these compounds.
Complete answer:
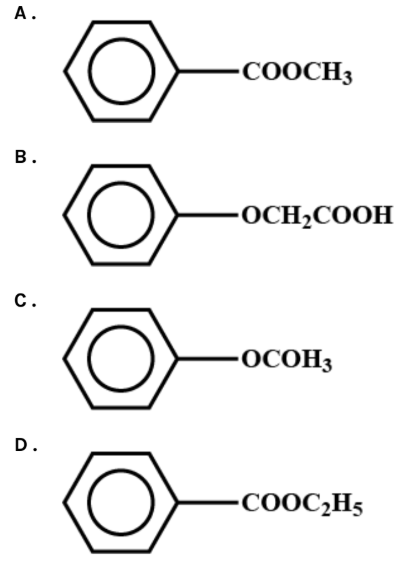
The compound structure as given in the first option corresponds to the methyl benzoate structure. Methyl benzoate is a compound organic to it. This ester has ${{\text{C}}_6}{{\text{H}}_5}{\text{COOC}}{{\text{H}}_3}$ as its chemical formula. It is a colorless liquid which is moderately water-soluble but miscible with organic solvents. Methyl benzoate has a good fragrance, highly reminiscent of the feijoa tree fruit and is used in perfumery. It is also used as a solvent and as a pesticide for attracting insects such as orchid bees.
The compound structure given in the second choice matches phenoxyacetic acid structure. Phenoxyacetic acid is a monocarboxylic acid, which is the glycolic acid derivative O-phenyl. A 2-phenoxyethanol metabolite, it is used in the manufacture of pharmaceuticals, pesticides , fungicides, and dyes. It has a role as a human xenobiotic metabolite, a metabolite of Aspergillus, a retardant for plant growth and an allergen. It is a monocarboxylic acid and a flavoring alcohol. It is derived from a glycolic acid. It is a phenoxyacetate Conjugate Acid.
The compound structure given in the third choice coincides with the phenyl ethanoate structure. Phenyl acetate is an acetate ester obtained by formal acetic acid condensation of the phenol. This is a phenyl acetate member, and a benzenes member. It originates from a phenol.
The structure of the compound given in the fourth option corresponds to the structure of ethyl benzoate. It is the ester which is formed by benzoic acid and ethanol condensation. This is an almost insoluble colorless liquid in water but miscible with other organic solvents. Ethyl benzoate, as with many volatile esters, has a pleasant odor described as sweet, winter-green, fruity, medicinal, cherry and grape. This is a part of many artificial fruit aromas and fragrances.
Therefore, option C is correct.
Note- Phenyl ethanoate and hydrogen chloride gas are formed together. Sometimes, first the phenol needs to be changed to make the reaction smoother. Benzoyl chloride, for example, has the formula ${{\text{C}}_6}{{\text{H}}_5}{\text{COCl}}$. The group -COCl is placed directly on a benzene ring. This is much less reactive than simple acyl chlorides such as ethanoyl chloride.
Complete answer:
The compound structure as given in the first option corresponds to the methyl benzoate structure. Methyl benzoate is a compound organic to it. This ester has ${{\text{C}}_6}{{\text{H}}_5}{\text{COOC}}{{\text{H}}_3}$ as its chemical formula. It is a colorless liquid which is moderately water-soluble but miscible with organic solvents. Methyl benzoate has a good fragrance, highly reminiscent of the feijoa tree fruit and is used in perfumery. It is also used as a solvent and as a pesticide for attracting insects such as orchid bees.
The compound structure given in the second choice matches phenoxyacetic acid structure. Phenoxyacetic acid is a monocarboxylic acid, which is the glycolic acid derivative O-phenyl. A 2-phenoxyethanol metabolite, it is used in the manufacture of pharmaceuticals, pesticides , fungicides, and dyes. It has a role as a human xenobiotic metabolite, a metabolite of Aspergillus, a retardant for plant growth and an allergen. It is a monocarboxylic acid and a flavoring alcohol. It is derived from a glycolic acid. It is a phenoxyacetate Conjugate Acid.
The compound structure given in the third choice coincides with the phenyl ethanoate structure. Phenyl acetate is an acetate ester obtained by formal acetic acid condensation of the phenol. This is a phenyl acetate member, and a benzenes member. It originates from a phenol.
The structure of the compound given in the fourth option corresponds to the structure of ethyl benzoate. It is the ester which is formed by benzoic acid and ethanol condensation. This is an almost insoluble colorless liquid in water but miscible with other organic solvents. Ethyl benzoate, as with many volatile esters, has a pleasant odor described as sweet, winter-green, fruity, medicinal, cherry and grape. This is a part of many artificial fruit aromas and fragrances.
Therefore, option C is correct.
Note- Phenyl ethanoate and hydrogen chloride gas are formed together. Sometimes, first the phenol needs to be changed to make the reaction smoother. Benzoyl chloride, for example, has the formula ${{\text{C}}_6}{{\text{H}}_5}{\text{COCl}}$. The group -COCl is placed directly on a benzene ring. This is much less reactive than simple acyl chlorides such as ethanoyl chloride.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




