
Which of the following is Polar:
A.\[N{F_3}\]
B.\[B{F_3}\]
C.\[S{F_4}\]
D.\[S{F_4}\]
Answer
583.2k+ views
Hint: Polarity is the separation of electric charges. In case of polar molecules individual bond moments will not cancel each other. For a non- polar molecule, individual bond moments will cancel out and net dipole moments will be zero.
Complete step by step answer:
Polarity is the separation of electric charges. The pair of bonding is not shared equally between two atoms. Such a bond is called a polar or heteropolar covalent bond.
Dipole moment is defined as the product of the magnitude of the charge and distance between the centers of positive and negative charge.
Dipole moment is represented by the symbol \[\mu \] .
\[\mu \] = Charge (Q) distance of separation (r).
For a polar molecule, individual bond moments will not cancel each other.
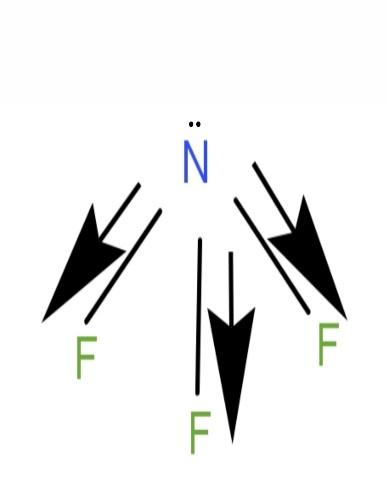
\[N{F_3}\] is a polar molecule. Its geometry is pyramidal. It has a lone pair of electrons on the nitrogen atom and has 3 bond pairs. N having a partial positive charge and F having a partial negative charge. N and F have different electronegativity. F is more electronegative than N. Dipole moment will be point in the direction of F. Dipole moment of \[N - F\] bonds will not cancel out and the molecule will have a net dipole moment.
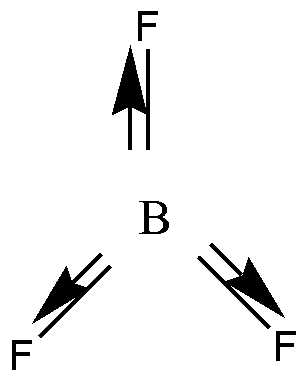
In \[B{F_3}\] , three bond pairs are present. B has three valence electrons, and it is shared with three F atoms. So \[B{F_3}\] has no lone pair of electrons. \[B{F_3}\] has trigonal planar geometry. B undergoes \[s{p^2}\] hybridization. Due to this planar shape individual bond moments will cancel each other. So \[B{F_3}\] is a nonpolar molecule.
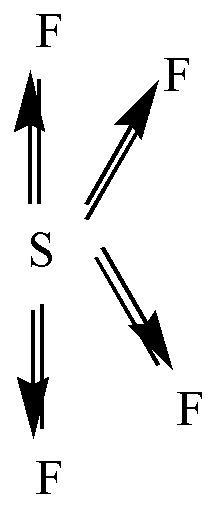
\[S{F_4}\] is a polar molecule. S has 6 valence electrons. Among these 4 electrons are shared with 4 F atoms and the remaining 2 electrons will stay as a lone pair. F is more electronegative so the dipole moment will point in the direction of F. Two of the \[S - F\] bonds are pointing away from each other and the other two dipoles are pointing down. So, the net dipole moment will not be zero.
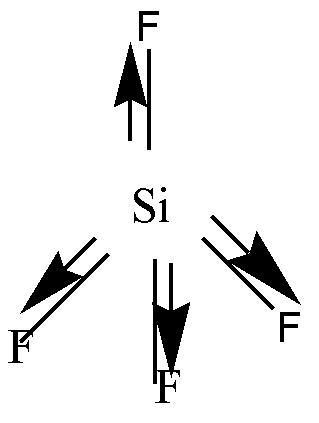
Si has 4 valence electrons. Each of these electrons is shared with 4 F atoms. So, the geometry will be tetrahedral. \[S - F\] bond is polar due to electronegativity difference. Due to the tetrahedral geometry individual moments will cancel each other. Hence it is a non-polar molecule.
Hence the correct answer is A and C. That is \[N{F_3}\] and \[S{F_4}\] .
Note: As the difference in the electronegativity between two covalently bonded atoms increases, the dipole character of the bond will also increase. Like \[Si{F_4}\] , \[SiC{l_4}\] and \[Si{H_4}\] are also non polar. In \[Si{F_4}\] , Si undergoes \[s{p^3}\] hybridization. In \[S{F_4}\] it undergoes \[s{p^3}\] d hybridization. Polar nature of water is responsible for its existence as a liquid at room temperature.
Complete step by step answer:
Polarity is the separation of electric charges. The pair of bonding is not shared equally between two atoms. Such a bond is called a polar or heteropolar covalent bond.
Dipole moment is defined as the product of the magnitude of the charge and distance between the centers of positive and negative charge.
Dipole moment is represented by the symbol \[\mu \] .
\[\mu \] = Charge (Q) distance of separation (r).
For a polar molecule, individual bond moments will not cancel each other.
\[N{F_3}\] is a polar molecule. Its geometry is pyramidal. It has a lone pair of electrons on the nitrogen atom and has 3 bond pairs. N having a partial positive charge and F having a partial negative charge. N and F have different electronegativity. F is more electronegative than N. Dipole moment will be point in the direction of F. Dipole moment of \[N - F\] bonds will not cancel out and the molecule will have a net dipole moment.

In \[B{F_3}\] , three bond pairs are present. B has three valence electrons, and it is shared with three F atoms. So \[B{F_3}\] has no lone pair of electrons. \[B{F_3}\] has trigonal planar geometry. B undergoes \[s{p^2}\] hybridization. Due to this planar shape individual bond moments will cancel each other. So \[B{F_3}\] is a nonpolar molecule.

\[S{F_4}\] is a polar molecule. S has 6 valence electrons. Among these 4 electrons are shared with 4 F atoms and the remaining 2 electrons will stay as a lone pair. F is more electronegative so the dipole moment will point in the direction of F. Two of the \[S - F\] bonds are pointing away from each other and the other two dipoles are pointing down. So, the net dipole moment will not be zero.

Si has 4 valence electrons. Each of these electrons is shared with 4 F atoms. So, the geometry will be tetrahedral. \[S - F\] bond is polar due to electronegativity difference. Due to the tetrahedral geometry individual moments will cancel each other. Hence it is a non-polar molecule.

Hence the correct answer is A and C. That is \[N{F_3}\] and \[S{F_4}\] .
Note: As the difference in the electronegativity between two covalently bonded atoms increases, the dipole character of the bond will also increase. Like \[Si{F_4}\] , \[SiC{l_4}\] and \[Si{H_4}\] are also non polar. In \[Si{F_4}\] , Si undergoes \[s{p^3}\] hybridization. In \[S{F_4}\] it undergoes \[s{p^3}\] d hybridization. Polar nature of water is responsible for its existence as a liquid at room temperature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




