
Which of the following is steam volatile?
(A) o-Nitrophenol
(B) p-nitrophenol
(C) Both of them
(D) Neither of them
Answer
590.7k+ views
Hint:
Any compound that can be distilled with steam distillation process, is called steam volatile compound. One of the compounds from o-nitrophenol and p-nitrophenol forms a special type of bond which lowers its melting and boiling point from the other.
Complete Step-by-Step Solution:
As shown in the hint part, steam volatile compound is a compound that can be distilled by steam distillation process. Now during steam distillation, the compound needs to have its melting point under the boiling point of water so that steam will pass through the solution and the compound will melt if it is in solid state and can be separated from impurities by distillation.
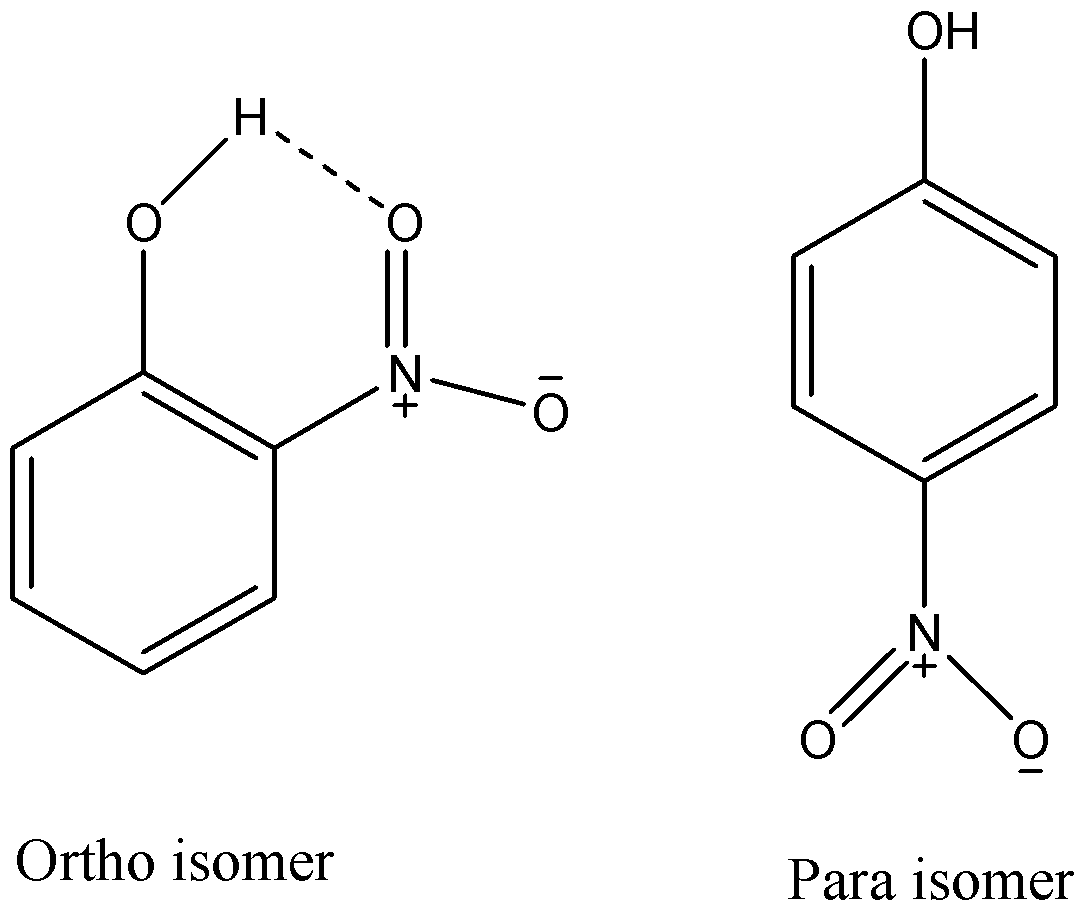
From the above given structures, we can see that ortho isomer has an intramolecular hydrogen bonding while para isomer cannot show it. As hydrogen atoms of o-nitrophenol are involved in intramolecular hydrogen bonding, they cannot effectively form hydrogen bonds with other molecules and hence it will have lower melting and boiling point than the para-isomer.
It is also practically proved that o-nitrophenol has M.P of \[{45^ \circ }C\] and p-nitrophenol has M.P of \[{119^ \circ }C\]. So, as o-nitrophenol has a lower melting point, it will easily melt and get converted to its vapours to get distilled by steam. While p-nitrophenol has a melting point higher than water’s boiling point and hence it will not be distilled with steam distillation. So, we can say that only o-nitrophenol is steam volatile.
Hence correct answer is (A) o-Nitrophenol
Note:
Remember that volatile compound and steam volatile compounds are totally different terminologies. Do not get confused with intramolecular and intermolecular hydrogen bonding as both are different and have different effects on the melting point of a compound.
Any compound that can be distilled with steam distillation process, is called steam volatile compound. One of the compounds from o-nitrophenol and p-nitrophenol forms a special type of bond which lowers its melting and boiling point from the other.
Complete Step-by-Step Solution:
As shown in the hint part, steam volatile compound is a compound that can be distilled by steam distillation process. Now during steam distillation, the compound needs to have its melting point under the boiling point of water so that steam will pass through the solution and the compound will melt if it is in solid state and can be separated from impurities by distillation.

From the above given structures, we can see that ortho isomer has an intramolecular hydrogen bonding while para isomer cannot show it. As hydrogen atoms of o-nitrophenol are involved in intramolecular hydrogen bonding, they cannot effectively form hydrogen bonds with other molecules and hence it will have lower melting and boiling point than the para-isomer.
It is also practically proved that o-nitrophenol has M.P of \[{45^ \circ }C\] and p-nitrophenol has M.P of \[{119^ \circ }C\]. So, as o-nitrophenol has a lower melting point, it will easily melt and get converted to its vapours to get distilled by steam. While p-nitrophenol has a melting point higher than water’s boiling point and hence it will not be distilled with steam distillation. So, we can say that only o-nitrophenol is steam volatile.
Hence correct answer is (A) o-Nitrophenol
Note:
Remember that volatile compound and steam volatile compounds are totally different terminologies. Do not get confused with intramolecular and intermolecular hydrogen bonding as both are different and have different effects on the melting point of a compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




