
Which of the following is the cofactor of carbonic anhydrase?
A. Fe
B. Zn
C. Cu
D. Mg
Answer
588.3k+ views
Hint: Carbonic anhydrase is classified as metalloenzymes due to the presence of metal ion which is a chemical element having atomic number 30. It belongs to group 12 of the periodic table as a first element.
Complete answer:
The other name of Carbonic anhydrase is carbonate dehydratases. It is an enzyme family that catalyzes the process of interconversion between carbon dioxide and water and helps in the dissociation of ions of the carbonic acid. Its active site consists of zinc ions for the reaction to occur.
Additional Information: -The enzyme maintains acid-base balance and helps in the transport of carbon dioxide.
-Its role changes according to its location and it maintains pH and fluid balance in the body parts.
-It is found in red blood cells, pancreatic cells, renal tubules, and gastric mucosa.
-Carbonic anhydrase is an old enzyme and was discovered in the year 1932.
-Its cofactor is zinc which helps in various physiological processes of higher vertebrates.
-A zinc prosthetic group is present in three positions by histidine side chains in carbonic anhydrase while the fourth one is occupied by water.
-It plays an important role in the closure of the stomata of the plant leaves.
So, the correct answer is ‘Zn’.
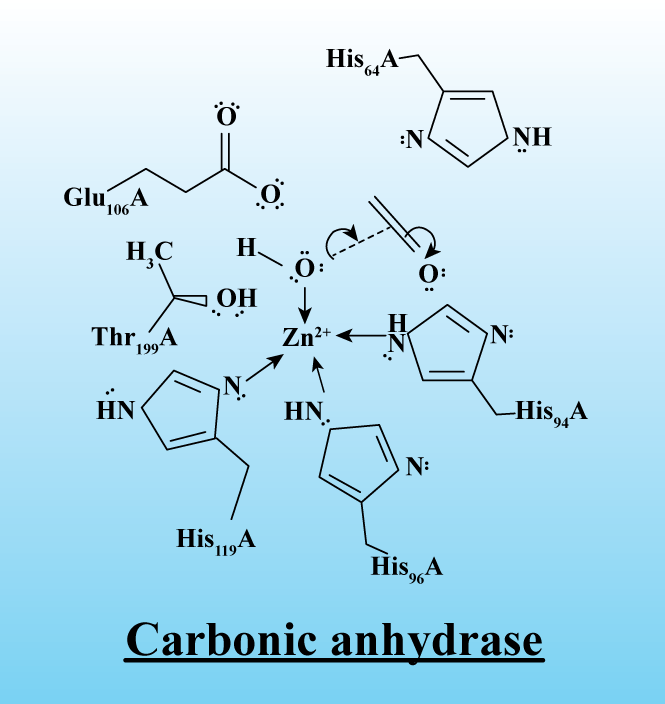
Note: Zinc is an essential element of the body and is present in higher concentrations in the case of the red blood cells. They are also present in the pancreas for the storage of insulin. It also protects the cell membranes from lysis due to the release of certain toxins. Zinc is taken from the diet we have to maintain nervous system functioning, cell growth, and immune health, and to develop wound healing mechanisms.
Complete answer:
The other name of Carbonic anhydrase is carbonate dehydratases. It is an enzyme family that catalyzes the process of interconversion between carbon dioxide and water and helps in the dissociation of ions of the carbonic acid. Its active site consists of zinc ions for the reaction to occur.
Additional Information: -The enzyme maintains acid-base balance and helps in the transport of carbon dioxide.
-Its role changes according to its location and it maintains pH and fluid balance in the body parts.
-It is found in red blood cells, pancreatic cells, renal tubules, and gastric mucosa.
-Carbonic anhydrase is an old enzyme and was discovered in the year 1932.
-Its cofactor is zinc which helps in various physiological processes of higher vertebrates.
-A zinc prosthetic group is present in three positions by histidine side chains in carbonic anhydrase while the fourth one is occupied by water.
-It plays an important role in the closure of the stomata of the plant leaves.
So, the correct answer is ‘Zn’.

Note: Zinc is an essential element of the body and is present in higher concentrations in the case of the red blood cells. They are also present in the pancreas for the storage of insulin. It also protects the cell membranes from lysis due to the release of certain toxins. Zinc is taken from the diet we have to maintain nervous system functioning, cell growth, and immune health, and to develop wound healing mechanisms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




