
Which of the following is the strongest base:
A.
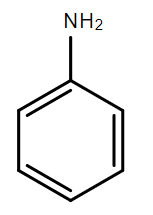
B.
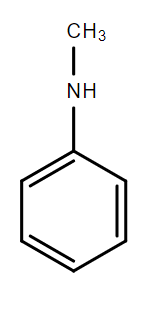
C.
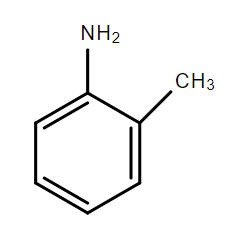
D.
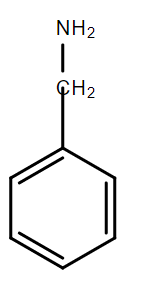




Answer
575.7k+ views
Hint: To answer this question, recall the concept of basic strength. Basic strength is defined as the ability of a base to donate the electrons. Use the concept of resonance and inductive effect to answer this question.
Complete step by step answer:
Unlike ammonia, amines can act as bases due to the presence of the lone pair of nitrogen electrons. Now the basicity of amines is affected majorly by inductive effect and resonance effect. We know that the resonance effect is more effective than inductive effect.
We can define the resonance effect as a chemical phenomenon which is observed in the characteristic compounds having double bonds in the organic compounds. We can define the inductive effect as when an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms (generally a carbon chain), the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect.
Presence of the electron-donating group increases the basicity of the amines whereas the electron-withdrawing group decreases the basicity.
In the compounds A, B and C, the lone pair of electrons on the nitrogen atom is in resonance with the aromatic ring and is not available for donation to an acid. Hence, these compounds are weak bases.
In the compound D, due to the presence of methylene group between the amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. Hence, this lone pair can be readily donated and benzylamine is the strongest base among the given bases.
Thus, the correct answer to this question is option D.
Note:
Relative inductive effects have been experimentally in laboratories with Hydrogen as the reference, the order of increasing $ + {\text{I}}$ effect is, as follows: \[N{H_3}^ + > N{O_2} > S{O_2}R > S{O_3}H > CHO > CO > COOH > COCl > CON{H_2} > F > Cl\]
\[ > Br > I > OH > OR > N{H_{2{\text{ }}}} > {C_6}{H_5} > CH = C{H_2} > H\]
Complete step by step answer:
Unlike ammonia, amines can act as bases due to the presence of the lone pair of nitrogen electrons. Now the basicity of amines is affected majorly by inductive effect and resonance effect. We know that the resonance effect is more effective than inductive effect.
We can define the resonance effect as a chemical phenomenon which is observed in the characteristic compounds having double bonds in the organic compounds. We can define the inductive effect as when an electron-releasing or an electron-withdrawing species is introduced to a chain of atoms (generally a carbon chain), the corresponding negative or positive charge is relayed through the carbon chain by the atoms belonging to it. This causes a permanent dipole to arise in the molecule and is referred to as the inductive effect.
Presence of the electron-donating group increases the basicity of the amines whereas the electron-withdrawing group decreases the basicity.
In the compounds A, B and C, the lone pair of electrons on the nitrogen atom is in resonance with the aromatic ring and is not available for donation to an acid. Hence, these compounds are weak bases.
In the compound D, due to the presence of methylene group between the amino group and the benzene ring, the resonance between the lone pair of electrons on N and the benzene ring is not possible. Hence, this lone pair can be readily donated and benzylamine is the strongest base among the given bases.
Thus, the correct answer to this question is option D.
Note:
Relative inductive effects have been experimentally in laboratories with Hydrogen as the reference, the order of increasing $ + {\text{I}}$ effect is, as follows: \[N{H_3}^ + > N{O_2} > S{O_2}R > S{O_3}H > CHO > CO > COOH > COCl > CON{H_2} > F > Cl\]
\[ > Br > I > OH > OR > N{H_{2{\text{ }}}} > {C_6}{H_5} > CH = C{H_2} > H\]
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




