
Which of the following molecule is polar:
A.
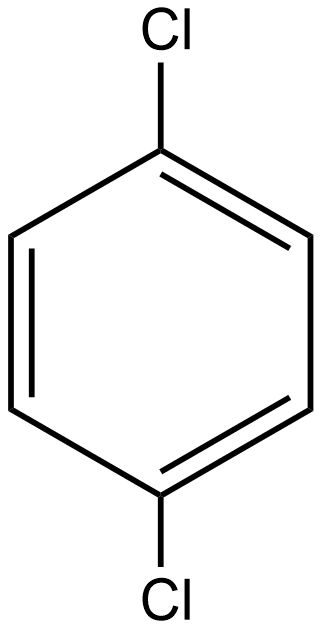
B.
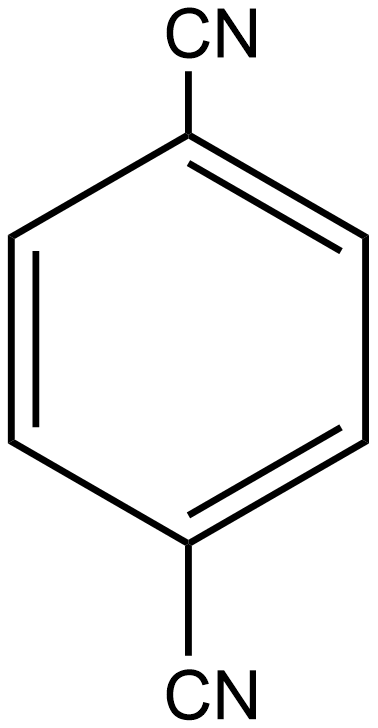
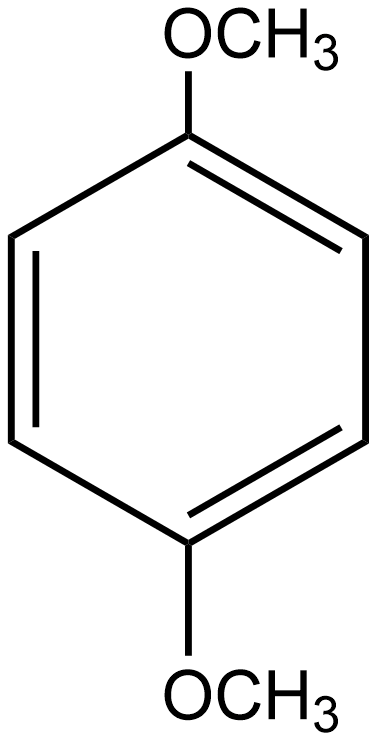
C.
D.All of these



Answer
575.4k+ views
Hint: Polar covalent compounds are defined as the chemical compounds that are held together by a polar covalent bond. The covalent bonds tend to have a polar nature due to the unequal sharing of electrons.
Complete step by step answer:
As we know the dipole moment is the measure of the polar nature of any molecule and is directly proportional to the polarity of the molecule. If we talk about option A i.e. p-dichlorobenzene the structure of the given compound is:
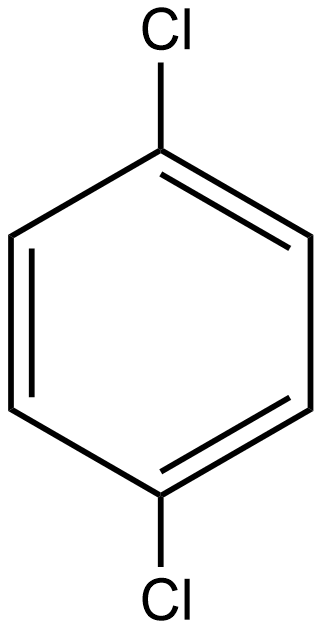
As we know the direction of dipole moment is positive to negative as shown below in the case of p-dichlorobenzene:
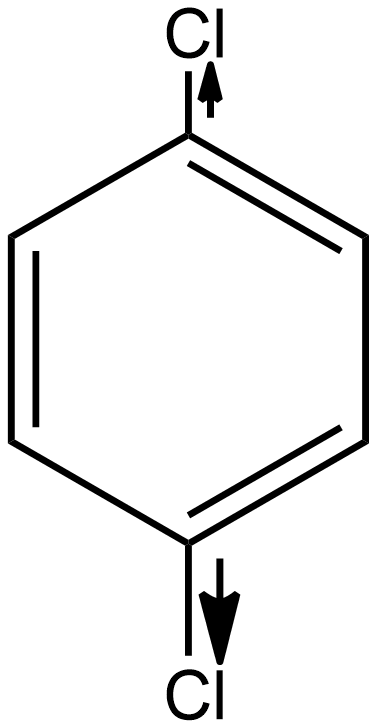
As we see in the above case the individual dipole moment is in the opposite direction so they cancel out each other as a result the resultant net dipole moment (μ)=0. Hence it is a non-polar compound.
Similarly, if we talk about option B i.e. p-dicyanobenzene, here again, the individual dipole moment is in the opposite direction as shown below:
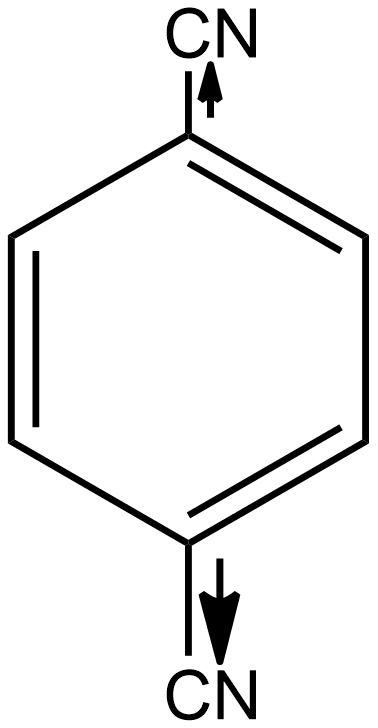
As a result, the individual dipole-moments cancel out each other. Thus the net resulting dipole-moment is again zero here. So we can say p-dicyanobenzene is also a non-polar compound.
In the case of option C i.e. p-dimethoxybenzene, the direction of dipole moment due to the highly electronegative nature of oxygen is shown below:
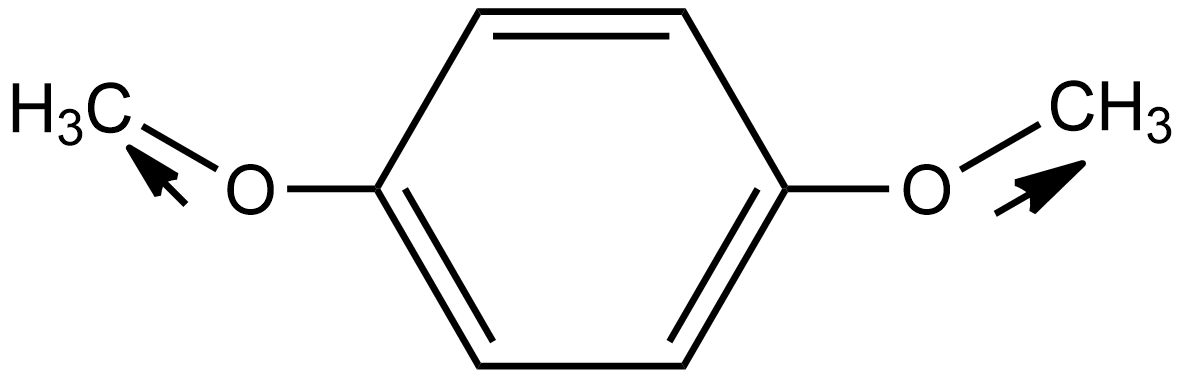
Hence in this case the dipole moment does not cancel out each other. Thus the net resulting dipole moment does not equal zero so we can say it is a polar molecule.
So, the correct answer is Option C.
Note: Dipole moment is also used to calculate the percentage of ionic character of a molecule and is also used to determine the symmetry of a molecule. The SI unit of dipole moment in debyes which is represented as “D”.
Complete step by step answer:
As we know the dipole moment is the measure of the polar nature of any molecule and is directly proportional to the polarity of the molecule. If we talk about option A i.e. p-dichlorobenzene the structure of the given compound is:

As we know the direction of dipole moment is positive to negative as shown below in the case of p-dichlorobenzene:

As we see in the above case the individual dipole moment is in the opposite direction so they cancel out each other as a result the resultant net dipole moment (μ)=0. Hence it is a non-polar compound.
Similarly, if we talk about option B i.e. p-dicyanobenzene, here again, the individual dipole moment is in the opposite direction as shown below:

As a result, the individual dipole-moments cancel out each other. Thus the net resulting dipole-moment is again zero here. So we can say p-dicyanobenzene is also a non-polar compound.
In the case of option C i.e. p-dimethoxybenzene, the direction of dipole moment due to the highly electronegative nature of oxygen is shown below:

Hence in this case the dipole moment does not cancel out each other. Thus the net resulting dipole moment does not equal zero so we can say it is a polar molecule.
So, the correct answer is Option C.
Note: Dipole moment is also used to calculate the percentage of ionic character of a molecule and is also used to determine the symmetry of a molecule. The SI unit of dipole moment in debyes which is represented as “D”.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




