
Which of the following molecules is least resonance stabilized?
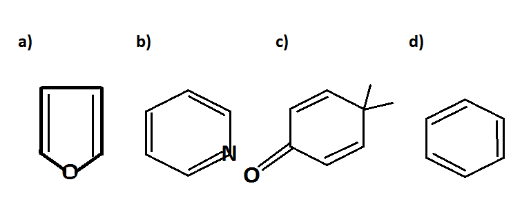

Answer
516.6k+ views
Hint :To solve this question we should know how the resonance takes place to draw the resonating structure and find out the correct answer. Resonance structures are sets of lewis structures that help to describe the displacement of electrons in a polyatomic ion or molecule.
Complete Step By Step Answer:
To find out the least stabilized resonance we will have to draw their resonating structure
Let us see the structures and find the answers;
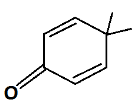
In Structure C resonance takes place when the double bond is shifted to oxygen, oxygen gets a negative sign and gives the following structure:
In structure D benzene resonance is possible overall giving the same structure as resonating structure.
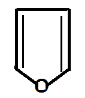
In structure A. it is called furan which is a heterocyclic compound. Heterocyclic compounds are those compounds where elements like oxygen, sulphur, nitrogen or any electronegative structure is fused in a ring. Furan on resonating gives the following structure;
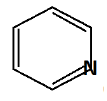
In the structure B pyridine the resonating structure is given as;
So from all the above resonating structures the; Structure a, b. and d resonates completely except structure c. So from all the structures only in structure the resonance is not complete. Hence the least stabilized structure is structure C.
Note :
Resonance can be considered as a way of describing a bonding in a particular atom or molecules or ions by merging many contributory structures or forms, called resonance structures within the theory of valence bonding into hybrid structure.
Complete Step By Step Answer:
To find out the least stabilized resonance we will have to draw their resonating structure
Let us see the structures and find the answers;
In Structure C resonance takes place when the double bond is shifted to oxygen, oxygen gets a negative sign and gives the following structure:

In structure D benzene resonance is possible overall giving the same structure as resonating structure.
In structure A. it is called furan which is a heterocyclic compound. Heterocyclic compounds are those compounds where elements like oxygen, sulphur, nitrogen or any electronegative structure is fused in a ring. Furan on resonating gives the following structure;

In the structure B pyridine the resonating structure is given as;

So from all the above resonating structures the; Structure a, b. and d resonates completely except structure c. So from all the structures only in structure the resonance is not complete. Hence the least stabilized structure is structure C.
Note :
Resonance can be considered as a way of describing a bonding in a particular atom or molecules or ions by merging many contributory structures or forms, called resonance structures within the theory of valence bonding into hybrid structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




