
Which of the following molecules shows intramolecular hydrogen bonding?
A. o- Nitrophenol
B. p- Nitrophenol
C. Benzoic acid
D. Ethanol
Answer
559.5k+ views
Hint: Intramolecular hydrogen bonds are those which occur within one molecule. This occurs when two functional groups of a molecule can form hydrogen bonds with each other. In order for this to happen, both a hydrogen donor and a hydrogen acceptor must be within one molecule and close to each other in the molecule.
Complete step by step answer:
Now we discuss the given options in detail.
First option is o- nitrophenol. The structure of ortho- nitrophenol is
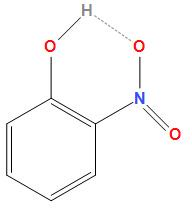
As we can see in the structure of ortho- nitrophenol. In ortho- nitrophenol the intramolecular hydrogen bonding takes place because of the $N{O_2}$ and $OH$ molecules are close to each other due to which there is a possibility of molecular association. The above structure shows the intramolecular hydrogen bonding in ortho- nitrophenol.
Second option is p- nitrophenol. The structure of para- nitrophenol is
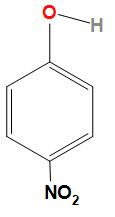
As we can see in the structure of para-nitrophenol. Both the $N{O_2}$ and $OH$ molecules are far from each other due to which intramolecular hydrogen bonding cannot take place.
Third option is Benzoic acid. The structure of benzoic acid is:
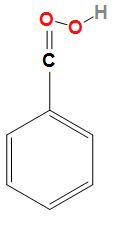
Here from the structure of benzoic acid we can conclude that no hydrogen bonding takes place due to the presence of one substituent which is the carboxylic acid group.
Fourth option is Ethanol. The structure of ethanol is:
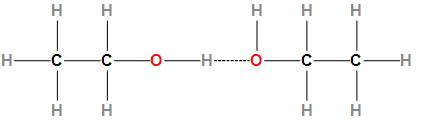
In ethanol intermolecular hydrogen bonding takes place but no intramolecular hydrogen bonding takes place.
So, the correct answer is Option A.
Note: Ortho- nitrophenol or 2- nitrophenol is used mainly as an intermediate for the production of dye stuffs, pigments, rubber chemicals and fungicides.
In small amounts ortho- nitrophenol is used as an acid base indicator and as a reagent for glucose
Complete step by step answer:
Now we discuss the given options in detail.
First option is o- nitrophenol. The structure of ortho- nitrophenol is

As we can see in the structure of ortho- nitrophenol. In ortho- nitrophenol the intramolecular hydrogen bonding takes place because of the $N{O_2}$ and $OH$ molecules are close to each other due to which there is a possibility of molecular association. The above structure shows the intramolecular hydrogen bonding in ortho- nitrophenol.
Second option is p- nitrophenol. The structure of para- nitrophenol is

As we can see in the structure of para-nitrophenol. Both the $N{O_2}$ and $OH$ molecules are far from each other due to which intramolecular hydrogen bonding cannot take place.
Third option is Benzoic acid. The structure of benzoic acid is:

Here from the structure of benzoic acid we can conclude that no hydrogen bonding takes place due to the presence of one substituent which is the carboxylic acid group.
Fourth option is Ethanol. The structure of ethanol is:

In ethanol intermolecular hydrogen bonding takes place but no intramolecular hydrogen bonding takes place.
So, the correct answer is Option A.
Note: Ortho- nitrophenol or 2- nitrophenol is used mainly as an intermediate for the production of dye stuffs, pigments, rubber chemicals and fungicides.
In small amounts ortho- nitrophenol is used as an acid base indicator and as a reagent for glucose
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




