
Which of the following orders is/are correct for the solvolysis in 50% aqueous ethanol at 44.6$^ \circ C$.
(A)
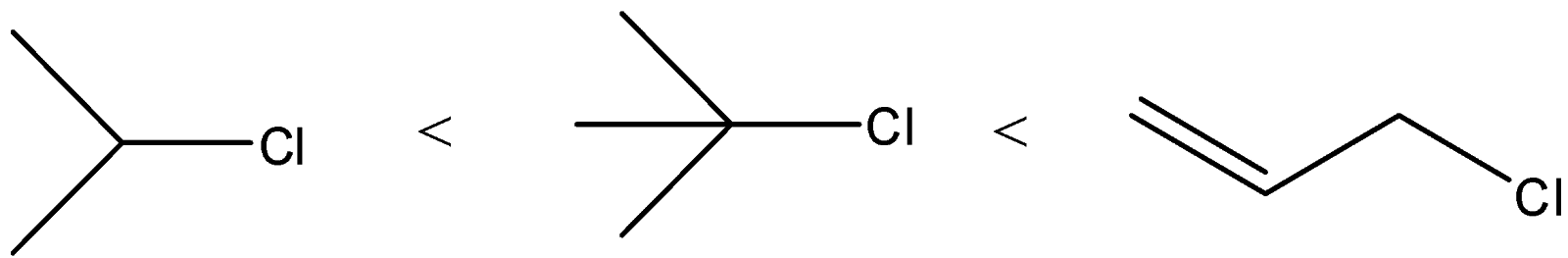
(B)
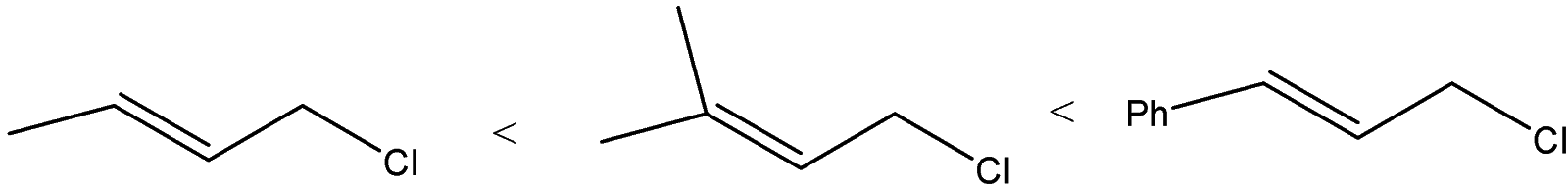
(C)
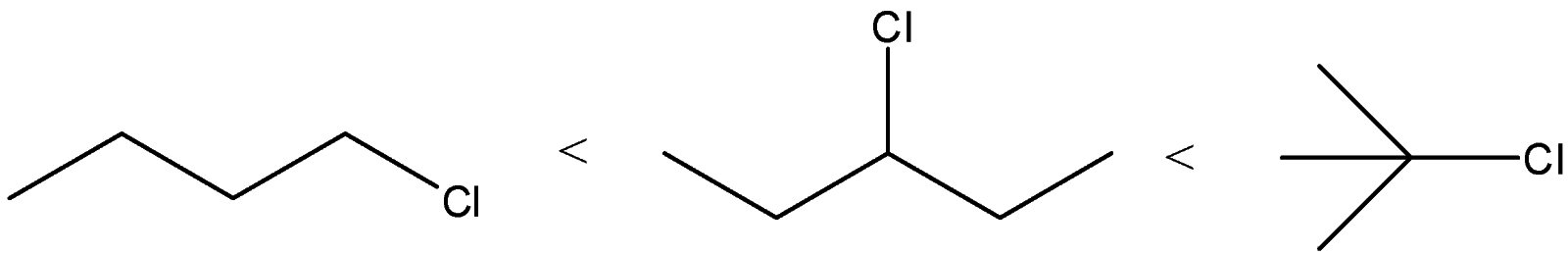
(D)
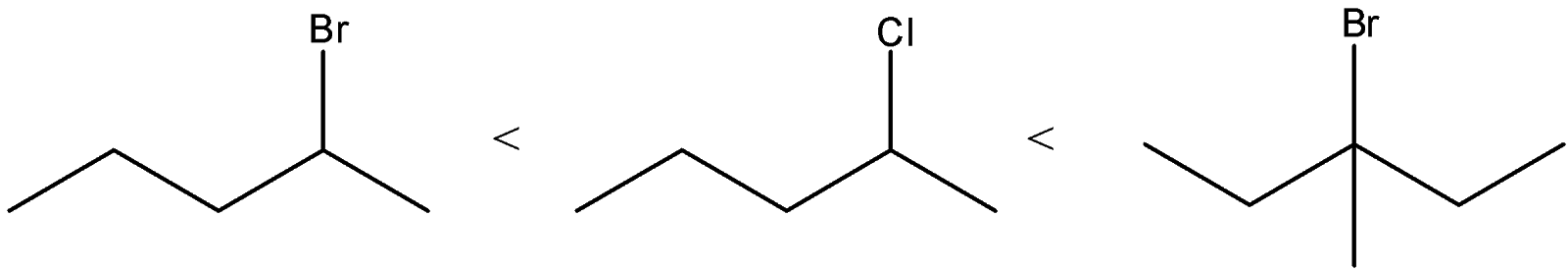




Answer
579k+ views
Hint: Solvolysis is a nucleophilic substitution reaction in which the solvent molecules act as nucleophiles. This reaction will go by ${S_N}1$ mechanism and so it will involve formation of carbocation.
Complete answer:
In this question, we need to find out which of the given orders are correct.
- Solvolysis is a nucleophilic substitution reaction in which the solvent molecule acts as a nucleophile.
- Here, the solvent is aqueous ethanol. We know that aqueous ethanol is polar in nature. So, it will favor ${S_N}1$ reaction.
- We know that ${S_N}1$ reaction involves formation of carbocation in the reaction. So, we can say that the compound which forms the most stable carbocation, will be favored more. Let’s analyze all the given options.
A) The correct order of the compounds given will be
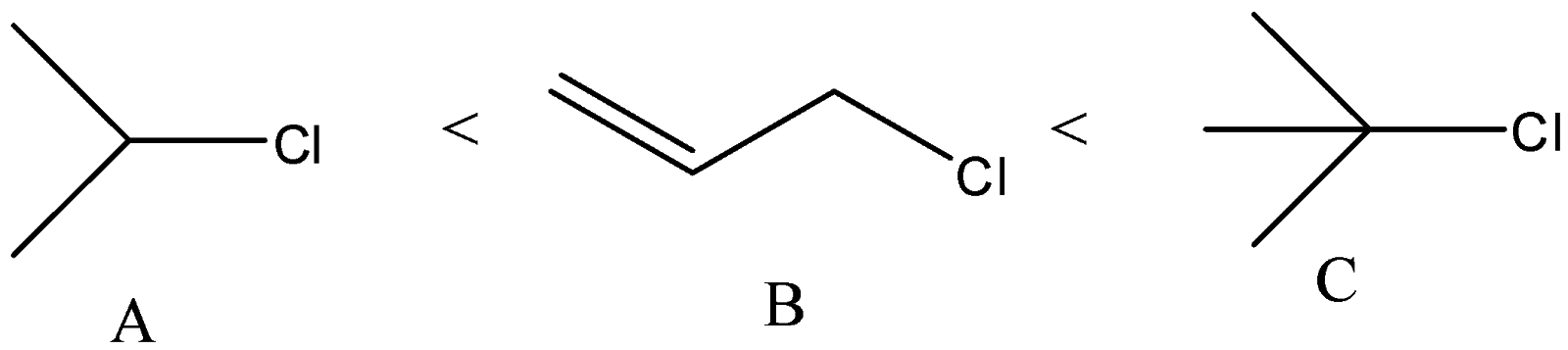
Here, we can see that the chlorine atom is bonded to a tertiary carbon atom. So, this will give the most stable carbocation. In compound B, the chlorine atom is an allylic position. It will form more stable carbocation than secondary carbocation and it will be less stable than tertiary. Secondary carbocation will be formed from compound A. So, it will be least favoured.
B) The order of the compounds given will be:
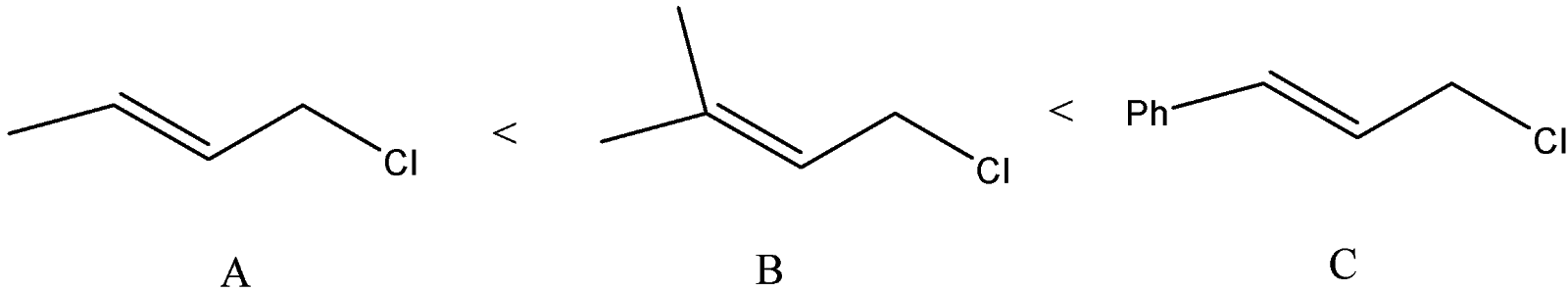
We can see that allylic carbocation will get formed as the chlorine atom will leave. The difference is the substitution at the other vinylic carbon atom. Phenyl group at the vinylic carbon stabilizes the carbocation. The substituted methyl groups will also stabilize the carbocation but compound B will form a more stable carbocation than A because A has only one methyl group.
C) The correct order of the compounds is:
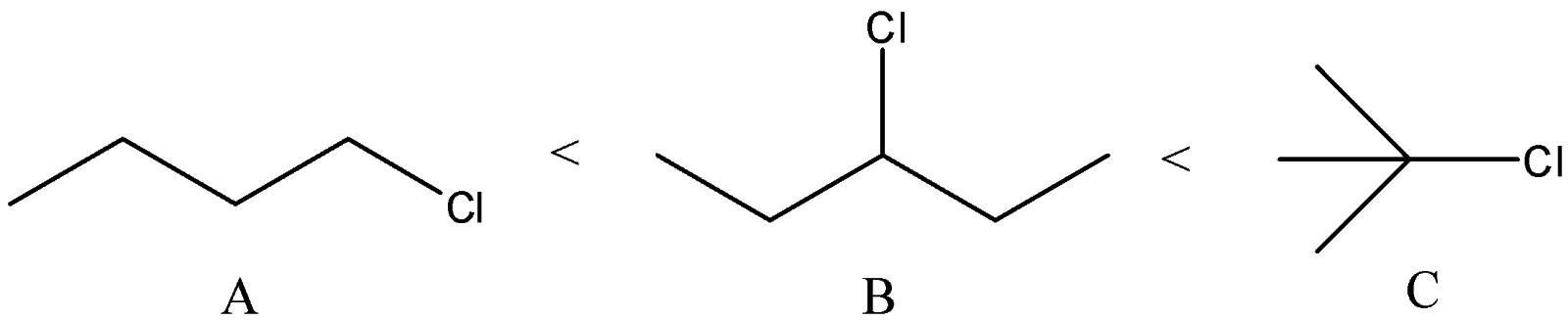
We know that order of the stability of carbocations is primary < secondary < allylic < tertiary
We can see that Compound A will give primary carbocation. Compound B will give secondary carbocation. Compound C will give tertiary carbocation. Thus, the order given is correct.
D) The correct order of solvolysis is given below.
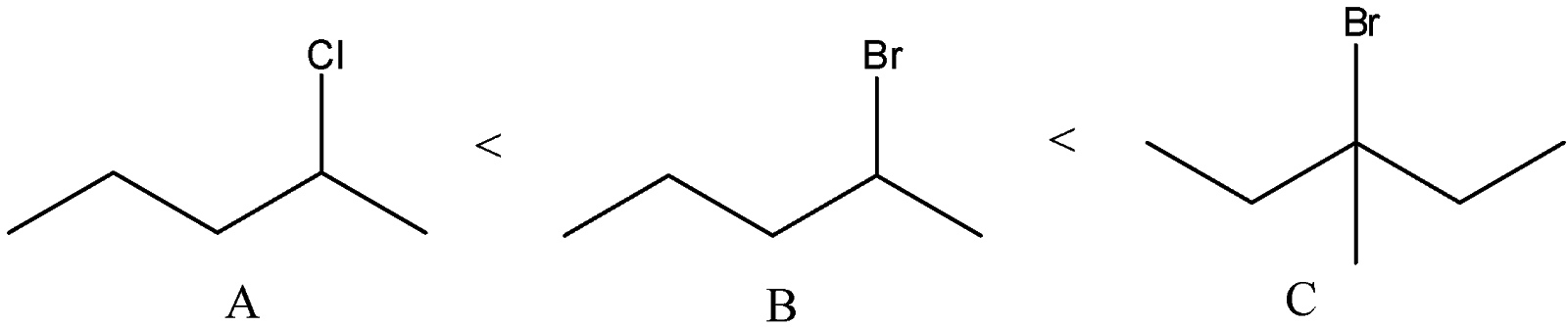
The compound C will give tertiary carbocation. So, it will be more favored. Compound A and B differ only by chlorine and bromine atom. Bromine atoms are a better leaving group than chlorine atoms. Thus, compound B will give solvolysis more easily than A.
Thus, we can conclude that the order given in option (B) and (C) is correct.
Note:
Here, the solvent given is aqueous ethanol. In polar solvents, ${S_N}1$ reactions are favored more. Aqueous ethanol is polar in nature. In non-polar solvents, ${S_N}2$ reactions are favored more than ${S_N}1$ mechanisms.
Complete answer:
In this question, we need to find out which of the given orders are correct.
- Solvolysis is a nucleophilic substitution reaction in which the solvent molecule acts as a nucleophile.
- Here, the solvent is aqueous ethanol. We know that aqueous ethanol is polar in nature. So, it will favor ${S_N}1$ reaction.
- We know that ${S_N}1$ reaction involves formation of carbocation in the reaction. So, we can say that the compound which forms the most stable carbocation, will be favored more. Let’s analyze all the given options.
A) The correct order of the compounds given will be

Here, we can see that the chlorine atom is bonded to a tertiary carbon atom. So, this will give the most stable carbocation. In compound B, the chlorine atom is an allylic position. It will form more stable carbocation than secondary carbocation and it will be less stable than tertiary. Secondary carbocation will be formed from compound A. So, it will be least favoured.
B) The order of the compounds given will be:

We can see that allylic carbocation will get formed as the chlorine atom will leave. The difference is the substitution at the other vinylic carbon atom. Phenyl group at the vinylic carbon stabilizes the carbocation. The substituted methyl groups will also stabilize the carbocation but compound B will form a more stable carbocation than A because A has only one methyl group.
C) The correct order of the compounds is:

We know that order of the stability of carbocations is primary < secondary < allylic < tertiary
We can see that Compound A will give primary carbocation. Compound B will give secondary carbocation. Compound C will give tertiary carbocation. Thus, the order given is correct.
D) The correct order of solvolysis is given below.

The compound C will give tertiary carbocation. So, it will be more favored. Compound A and B differ only by chlorine and bromine atom. Bromine atoms are a better leaving group than chlorine atoms. Thus, compound B will give solvolysis more easily than A.
Thus, we can conclude that the order given in option (B) and (C) is correct.
Note:
Here, the solvent given is aqueous ethanol. In polar solvents, ${S_N}1$ reactions are favored more. Aqueous ethanol is polar in nature. In non-polar solvents, ${S_N}2$ reactions are favored more than ${S_N}1$ mechanisms.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




